Stress Impairs Prefrontal Cortical Function via D1 Dopamine Receptor Interactions With Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels - PubMed (original) (raw)
. 2015 Dec 15;78(12):860-70.
doi: 10.1016/j.biopsych.2015.01.009. Epub 2015 Feb 4.
Gyorgy Lur 2, Michael J Higley 2, Min Wang 3, Constantinos D Paspalas 3, Susheel Vijayraghavan 3, Yang Yang 3, Brian P Ramos 3, Kathy Peng 3, Anna Kata 3, Lindsay Boven 3, Faith Lin 3, Lisette Roman 3, Daeyeol Lee 3, Amy F T Arnsten 3
Affiliations
- PMID: 25731884
- PMCID: PMC4524795
- DOI: 10.1016/j.biopsych.2015.01.009
Stress Impairs Prefrontal Cortical Function via D1 Dopamine Receptor Interactions With Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels
Nao J Gamo et al. Biol Psychiatry. 2015.
Abstract
Background: Psychiatric disorders such as schizophrenia are worsened by stress, and working memory deficits are often a central feature of illness. Working memory is mediated by the persistent firing of prefrontal cortical (PFC) pyramidal neurons. Stress impairs working memory via high levels of dopamine D1 receptor (D1R) activation of cyclic adenosine monophosphate signaling, which reduces PFC neuronal firing. The current study examined whether D1R-cyclic adenosine monophosphate signaling reduces neuronal firing and impairs working memory by increasing the open state of hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels, which are concentrated on dendritic spines where PFC pyramidal neurons interconnect.
Methods: A variety of methods were employed to test this hypothesis: dual immunoelectron microscopy localized D1R and HCN channels, in vitro recordings tested for D1R actions on HCN channel current, while recordings in monkeys performing a working memory task tested for D1R-HCN channel interactions in vivo. Finally, cognitive assessments following intra-PFC infusions of drugs examined D1R-HCN channel interactions on working memory performance.
Results: Immunoelectron microscopy confirmed D1R colocalization with HCN channels near excitatory-like synapses on dendritic spines in primate PFC. Mouse PFC slice recordings demonstrated that D1R stimulation increased HCN channel current, while local HCN channel blockade in primate PFC protected task-related firing from D1R-mediated suppression. D1R stimulation in rat or monkey PFC impaired working memory performance, while HCN channel blockade in PFC prevented this impairment in rats exposed to either stress or D1R stimulation.
Conclusions: These findings suggest that D1R stimulation or stress weakens PFC function via opening of HCN channels at network synapses.
Keywords: D(1) dopamine receptor; HCN channel; Prefrontal cortex; Stress; Working memory; cAMP.
Copyright © 2015 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosures: All authors declare no biomedical financial interests or potential conflicts of interest.
Figures
Figure 1
Single-unit recording from monkey dorsolateral prefrontal cortex (DLPFC) was combined with drug iontophoresis in monkeys performing the oculomotor delayed response (ODR) task. A. The ODR task, a test of spatial working memory. The monkey was seated in front of a screen, and the trial began when he fixated on a central target on the screen for 0.5s (fixation period). Next, a cue appeared briefly (0.5s) in one of eight peripheral locations on the screen (cue period), followed by a 2.5s delay period, during which the monkey continued to maintain fixation. At the end of the delay period, the fixation target was extinguished, and the monkey was required to make a memory-guided saccade to the remembered location of the cue (response period); monkeys were rewarded with a drop of juice for each correct response. Each test session consisted of hundreds of trials across which the cued location randomly changed, thus requiring the monkey to update his working memory. The TEMPO Experiment Control System (Reflective Computing, St. Louis, MO) generated the task, while the ISCAN Eye Movement Monitoring System (ISCAN Inc., Woburn, MA) monitored eye position. B. Single-unit recording was performed in DLPFC. AS: arcuate sulcus; PS: principal sulcus. C. Firing patterns of a sample neuron from the current study. Under optimal conditions, neurons show delay-related firing for a preferred direction (e.g. 225°), but suppress firing for non-preferred directions (2). The dark gray background indicates the cue period, and the light gray background indicates the delay period. D. Circuit basis for spatial working memory (2). Spatial working memory is maintained in DLPFC by recurrent excitation among networks of NMDA-receptor (NMDA-R) glutamatergic pyramidal neurons with shared stimulus inputs (e.g. 225°). Spatial tuning is enhanced by lateral inhibition of non-preferred inputs (e.g. 45°) from gamma-aminobutyric acidergic (GABAergic) interneurons (16). E. Working model of molecular mechanisms that weaken PFC network connectivity. Cyclic adenosine monophosphate (cAMP) directly increases the open probability of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, while ZD7288 (ZD) blocks them. Dynamic Network Connectivity signaling proteins are often found in long, thin spines with narrow spine necks at NR2B NMDA-R synapses in layer III monkey DLPFC (2). AC: adenylyl cyclase.
Figure 2
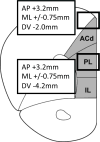
Drug infusions into rat prelimbic PFC. Rats were implanted with chronic infusion cannulae directed above prelimbic PFC (PL) (AP +3.2mm; ML +/−0.75mm; DV −4.2mm) or dorsal anterior cingulate cortex (ACd) (AP +3.2mm; ML +/−0.75mm; DV −2.0mm), as indicated by boxes. IL: infralimbic cortex.
Figure 3
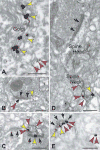
Dual immunoelectron microscopy for HCN1 channels and dopamine (DA) D1 receptors (D1Rs) in monkey DLPFC. A. HCN1 channels (red arrows) and D1Rs (yellow arrows) were co-expressed in layer III pyramidal neurons in the Golgi and reticular endomembranes, showing that D1R are manufactured by layer III pyramidal cells. B–E. In the neuropil, HCN1 channels and D1Rs were colocalized in dendritic spines, as shown by gold-gold (B) or peroxidase-gold (C–E) labeling. HCN1 channels were localized at the elongated spine neck with D1Rs positioned at the base of the emerging spine (D). An oblique section through a synapse (the synaptic disk is indicated by multiple arrows) demonstrated perisynaptic localization for both proteins (E); lead-contrasting was omitted from E to facilitate visualization of D1R-immunoperoxidase. Black arrows point to axospinous synapses. Scale bars: 200nm.
Figure 4
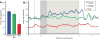
The D1R agonist, SKF38393 (SKF), suppressed delay-related firing, and this suppression was blocked by the HCN channel blocker, ZD, in monkeys performing the ODR task (N = 16 neurons). A. Drug effects on the mean firing rate during the delay period. Error bars represent SEM. *p < 0.0005 vs. Control, ZD+SKF. B. Drug effects on population firing across all task epochs. The dotted line indicates onset of the fixation period, the dark grey background indicates the cue period, and the light grey background indicates the delay period.
Figure 5
In the subset of neurons in which we assessed ZD alone and Recovery, SKF38393 (SKF) suppressed delay-related firing, and this suppression was blocked by a non-improving dose of ZD in monkeys performing the ODR task (N = 6). Firing increased during Recovery, following the removal of SKF. A. Drug effects on the mean firing rate during the delay period. Error bars represent SEM. *p < 0.0005 vs. Control, ZD+SKF; †p = 0.030 vs. SKF. B. Drug effects on population firing across all task epochs. The dotted line indicates onset of the fixation period, the dark grey background indicates the cue period, and the light grey background indicates the delay period.
Figure 6
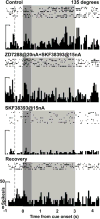
The effects of SKF38393 (SKF) and ZD on neuronal firing in an individual neuron recorded in DLPFC while a monkey performed the ODR task. For the 135° direction, the neuron showed enhanced delay-related firing (p = 0.0020 vs. fixation) during Control. The delay firing was maintained with ZD+SKF (p > 0.05 vs. Control), but was then reduced with SKF (p = 0.00017 vs. Control; 0.0015 vs. ZD+SKF). The delay firing returned during Recovery (p = 0.00013 vs. SKF). The ZD condition was not performed in this neuron. The dotted line indicates onset of the fixation period, the dark grey background indicates the cue period, and the light grey background indicates the delay period.
Figure 7
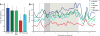
The effects of the D1R agonist, SKF81297 (SKF), on the HCN channel current (Ih) in layer V pyramidal neurons in mouse PFC slices. A. SKF increased the ratio of the maximum change in membrane potential to the subsequent steady state potential during the hyperpolarizing pulse (*p = 0.038 vs. Control; N = 7). Error bars represent SEM. B. A sample trace showing that SKF induced a “sag” in the membrane potential relative to control conditions, which indicated an increase in Ih. C. The trace in B scaled to highlight the difference in the membrane potential response between SKF and control conditions.
Figure 8
The effects of SKF81297 (SKF) and stress on spatial working memory performance in rats. A. SKF impaired spatial working memory performance in rats (N = 6) relative to vehicle (Veh) (*p = 0.010 vs. Veh+Veh), and this impairment was blocked by PFC infusions of ZD (†p=0.001 vs. SKF+Veh). Results represent % correct out of 10 trials. Error bars represent SEM. B. The pharmacological stressor, FG7142 (FG) impaired spatial working memory performance in rats (N = 5) relative to vehicle (Veh) (*p = 0.004 vs. Veh+Veh), and this impairment was blocked by PFC infusions of ZD (†p = 0.006 vs. FG+Veh). Results represent % correct out of 10 trials. Error bars represent SEM. C. FG impaired spatial working memory performance in rats (N = 7) relative to vehicle (Veh) (*p = 0.002 vs. Veh+Veh). However, this impairment was not blocked by ZD infusions dorsal to PFC (†p = 0.008 vs. Veh+Veh). Results represent % correct out of 10 trials. Error bars represent SEM.
Similar articles
- Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex: potential substrate for working memory deficits in schizophrenia.
Paspalas CD, Wang M, Arnsten AF. Paspalas CD, et al. Cereb Cortex. 2013 Jul;23(7):1643-54. doi: 10.1093/cercor/bhs152. Epub 2012 Jun 12. Cereb Cortex. 2013. PMID: 22693343 Free PMC article. - Interaction Between HCN and Slack Channels Regulates mPFC Pyramidal Cell Excitability in Working Memory Circuits.
Wu J, El-Hassar L, Datta D, Thomas M, Zhang Y, Jenkins DP, DeLuca NJ, Chatterjee M, Gribkoff VK, Arnsten AFT, Kaczmarek LK. Wu J, et al. Mol Neurobiol. 2024 Apr;61(4):2430-2445. doi: 10.1007/s12035-023-03719-8. Epub 2023 Oct 27. Mol Neurobiol. 2024. PMID: 37889366 - Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex.
Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Wang M, et al. Cell. 2007 Apr 20;129(2):397-410. doi: 10.1016/j.cell.2007.03.015. Cell. 2007. PMID: 17448997 - Dopamine's Actions in Primate Prefrontal Cortex: Challenges for Treating Cognitive Disorders.
Arnsten AF, Wang M, Paspalas CD. Arnsten AF, et al. Pharmacol Rev. 2015 Jul;67(3):681-96. doi: 10.1124/pr.115.010512. Pharmacol Rev. 2015. PMID: 26106146 Free PMC article. Review. - Neurophysiology of HCN channels: from cellular functions to multiple regulations.
He C, Chen F, Li B, Hu Z. He C, et al. Prog Neurobiol. 2014 Jan;112:1-23. doi: 10.1016/j.pneurobio.2013.10.001. Epub 2013 Oct 29. Prog Neurobiol. 2014. PMID: 24184323 Review.
Cited by
- Guanfacine's mechanism of action in treating prefrontal cortical disorders: Successful translation across species.
Arnsten AFT. Arnsten AFT. Neurobiol Learn Mem. 2020 Dec;176:107327. doi: 10.1016/j.nlm.2020.107327. Epub 2020 Oct 17. Neurobiol Learn Mem. 2020. PMID: 33075480 Free PMC article. Review. - Receptor-Receptor Interactions as a Widespread Phenomenon: Novel Targets for Drug Development?
Guidolin D, Marcoli M, Tortorella C, Maura G, Agnati LF. Guidolin D, et al. Front Endocrinol (Lausanne). 2019 Feb 18;10:53. doi: 10.3389/fendo.2019.00053. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30833931 Free PMC article. Review. - Localization of PDE4D, HCN1 channels, and mGluR3 in rhesus macaque entorhinal cortex may confer vulnerability in Alzheimer's disease.
Datta D, Perone I, Morozov YM, Arellano J, Duque A, Rakic P, van Dyck CH, Arnsten AFT. Datta D, et al. Cereb Cortex. 2023 Dec 9;33(24):11501-11516. doi: 10.1093/cercor/bhad382. Cereb Cortex. 2023. PMID: 37874022 Free PMC article. - Behavioral Neuroadaptation to Alcohol: From Glucocorticoids to Histone Acetylation.
Mons N, Beracochea D. Mons N, et al. Front Psychiatry. 2016 Oct 6;7:165. doi: 10.3389/fpsyt.2016.00165. eCollection 2016. Front Psychiatry. 2016. PMID: 27766083 Free PMC article. Review. - The Effects of Stress Exposure on Prefrontal Cortex: Translating Basic Research into Successful Treatments for Post-Traumatic Stress Disorder.
Arnsten AF, Raskind MA, Taylor FB, Connor DF. Arnsten AF, et al. Neurobiol Stress. 2015 Jan 1;1:89-99. doi: 10.1016/j.ynstr.2014.10.002. Neurobiol Stress. 2015. PMID: 25436222 Free PMC article.
References
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):i6–15. - PubMed
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. - PubMed
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 NS007224/NS/NINDS NIH HHS/United States
- MH099045/MH/NIMH NIH HHS/United States
- NS07224/NS/NINDS NIH HHS/United States
- U54RR024350/RR/NCRR NIH HHS/United States
- R01 MH099045/MH/NIMH NIH HHS/United States
- RL1AA017536/AA/NIAAA NIH HHS/United States
- RL1 AA017536/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous