Modification of Helicobacter pylori Peptidoglycan Enhances NOD1 Activation and Promotes Cancer of the Stomach - PubMed (original) (raw)
Modification of Helicobacter pylori Peptidoglycan Enhances NOD1 Activation and Promotes Cancer of the Stomach
Giovanni Suarez et al. Cancer Res. 2015.
Abstract
Helicobacter pylori (H. pylori) is the strongest known risk factor for gastric carcinogenesis. One cancer-linked locus is the cag pathogenicity island, which translocates components of peptidoglycan into host cells. NOD1 is an intracellular immune receptor that senses peptidoglycan from Gram-negative bacteria and responds by inducing autophagy and activating NF-κB, leading to inflammation-mediated bacterial clearance; however chronic pathogens can evade NOD1-mediated clearance by altering peptidoglycan structure. We previously demonstrated that the H. pylori cag(+) strain 7.13 rapidly induces gastric cancer in Mongolian gerbils. Using 2D-DIGE and mass spectrometry, we identified a novel mutation within the gene encoding the peptidoglycan deacetylase PgdA; therefore, we sought to define the role of H. pylori PgdA in NOD1-dependent activation of NF-κB, inflammation, and cancer. Coculture of H. pylori strain 7.13 or its pgdA(-) isogenic mutant with AGS gastric epithelial cells or HEK293 epithelial cells expressing a NF-κB reporter revealed that pgdA inactivation significantly decreased NOD1-dependent NF-κB activation and autophagy. Infection of Mongolian gerbils with an H. pylori pgdA(-) mutant strain led to significantly decreased levels of inflammation and malignant lesions in the stomach; however, preactivation of NOD1 before bacterial challenge reciprocally suppressed inflammation and cancer in response to wild-type H. pylori. Expression of NOD1 differs in human gastric cancer specimens compared with noncancer samples harvested from the same patients. These results indicate that peptidoglycan deacetylation plays an important role in modulating host inflammatory responses to H. pylori, allowing the bacteria to persist and induce carcinogenic consequences in the gastric niche.
©2015 American Association for Cancer Research.
Conflict of interest statement
Conflicts of Interest: None
Figures
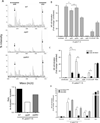
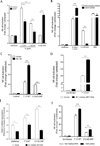
Figure 1. Inactivation of pgdA affects peptidoglycan acetylation and attenuates NOD1-dependent NF-κB activation induced by H. pylori in vitro
Peptidoglycan from WT strain 7.13, a pgdA− mutant, or a complemented pgdA (pgdA/c) strain was analyzed by mass spectrometry and ratios between m/z 1470 (de-acetylated) and m/z 1512 (acetylated) were calculated (A). AGS cells transfected with a NF-κB luciferase reporter (B), co-transfected with a dominant negative NOD1 (DN NOD1) (C), or transfected with shRNA targeting NOD1 or NOD2 (D) were co-cultured with WT strain 7.13, a pgdA− mutant, a pgdA complemented mutant (pgdA/c), a cagA− mutant, or a cagE− mutant for 4 hours. C12-iE-DAP and PMA were used as NOD1-dependent and NOD1-independent activators of NF-κB, respectively. (***:p≤0.001;**:p≤0.01;*:p≤0.05) (+:p≤0.05 versus uninfected control). Data represent mean±SEM from at least 3 experiments.
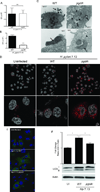
Figure 2. Inactivation of pgdA sensitizes H. pylori to intracellular degradation but not autophagy
A. Co-cultures of H. pylori and AGS cells were lysed and plated to quantify adherent bacteria. Data represent mean±SEM from at least 3 experiments (NS:Non significant). B. Gentamycin protection assays were performed to quantify viable intracellular H. pylori. (***:p≤0.001). Data represent mean±SEM from at least 3 experiments. C. Transmission electron microscopy of WT strain 7.13 or a pgdA− mutant co-cultured with AGS cells for 4 hours. Arrows: bacteria; arrowheads: phagolysosomal vesicles. D. Confocal images of AGS cells co-cultured with WT 7.13 strain or a pgdA− mutant for 4 hours. Cells were stained with LysoTracker DND-99 (red) and nuclear stain Hoechst 33342 (white). E. Confocal images of AGS cells expressing a GFP-LC3 fusion protein (green) co-cultured with WT strain 7.13 or its pgdA− mutant for 6 hours. Cells were fixed and mounted in media containing DAPI (Blue). F. Western blot of AGS cells co-cultured with WT strain 7.13 or its pgdA− mutant using anti-LC3 and anti-actin antibodies, and subsequent densitometry analysis from three replicates (*:p≤0.05).
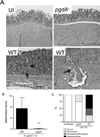
Figure 3. Loss of H. pylori pgdA decreases colonization in Mongolian gerbils
A. Colonization efficiency of H. pylori strains in gerbils. Two and twelve week post-challenge results are combined (*:p≤0.05). B. Silver-stain images from gerbils infected with WT or pgdA− mutant strains, 12 weeks post-challenge. C. Colonization density of WT or pgdA− strains 12 weeks post-challenge (*:p≤0.05). D. _H. pylori_-specific antibodies were quantified by ELISA in sera harvested from gerbils challenged with WT or pgdA− mutant 7.13 strains for 12 weeks (*:p≤0.05).
Figure 4. Inactivation of PgdA alters _H. pylori_-induced inflammation and disease outcome in Mongolian gerbils
A. H&E-stained gastric tissue from gerbils challenged with WT strain 7.13 or a pgdA− mutant strain for 12 weeks. UI: uninfected. Bottom left, severe gastritis (arrow); bottom right, invasive adenocarcinoma (arrow). B. Inflammatory scores in gerbils infected with H. pylori 12 weeks post-infection (***:p≤0.001); data represent mean±SEM. C. Disease outcome in gerbils challenged with 7.13 WT or its pgdA− mutant, 12 weeks post-challenge.
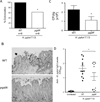
Figure 5. Pre-activation of NOD1 in Mongolian gerbils prior to H. pylori infection attenuates disease
A. Gerbils were treated with different doses of C12-iE-DAP for 6 days. Expression of KC (Cxcl1) at day seven was measured by RT-PCR as an indicator of NOD1 activation. Data represent mean±SEM. Colonization density (B), inflammation (C), and incidence of adenocarcinoma (D) in gerbils treated with C12-iE-DAP (40µg/animal/day) for 3 days before and 3 days after challenge with WT strain 7.13. Animals were sacrificed 12 weeks post-challenge. (**:p≤0.01;*:p≤0.05).
Figure 6. Overexpression or inhibition of NOD1 prior to H. pylori infection alters NF-κB activation
HEK293 cells carrying a NF-κB alkaline phosphatase-linked reporter and co-transfected with a human NOD1-overexpression vector (pUNO1-hNOD1) or a control construct (Control) were purchased and co-cultured with strains 7.13 or J166 for 24 hours. Supernatants were harvested and alkaline phosphatase activity was quantified (A). AGS cells were stably transfected with a NF-κB-Luciferase linked reporter and either non-targeting shRNA or shRNA specific for NOD1 (B) or TRAF3 (F) or pre-treated for 3 days with ML130, a specific inhibitor of NOD1 activation (C), or SR11302, a specific inhibitor of AP-1 (D). Luciferase activity was measured after 4 hours of co-culture with H. pylori. TRAF3 mRNA expression was measured by real-time RT-PCR in AGS cells pre-treated with SR11302 for 3 days and then challenged with WT strain 7.13 for 4 hours (E). Data represent mean±SEM from at least 3 experiments. (***:p≤0.001;**:p≤0.01;*:p≤0.05)(+: p≤0.05 compared with uninfected cells).
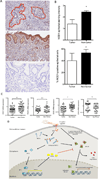
Figure 7. Expression of NOD1 is diminished in human malignant tissue
A. Immunohistochemistry for NOD1 in tumoral (red) and non-tumoral epithelia (middle panel) compared with isotype control (bottom panel). B. NOD1 staining intensity in epithelial cells (top panel) and percentage of NOD1-positive epithelial cells (bottom panel). Data represent mean±SEM. (*:p≤0.05). C. Quantitative RT-PCR expression for NOD1 and related genes in gastric biopsies from tumoral and non-tumoral tissue (***:p≤0.001;**:p≤0.01;*:p≤0.05). Results expressed as ratio of target gene/GAPDH mRNA in cancer or non-cancer samples relative to the mean ratio in non-tumor tissue. Data represent mean±SEM from 3 replicates/sample. D. Working model of NOD1 activation and downstream consequences within the context of H. pylori infection.
Similar articles
- Nucleotide-binding oligomerization domain 1 and Helicobacter pylori infection: A review.
Minaga K, Watanabe T, Kamata K, Asano N, Kudo M. Minaga K, et al. World J Gastroenterol. 2018 Apr 28;24(16):1725-1733. doi: 10.3748/wjg.v24.i16.1725. World J Gastroenterol. 2018. PMID: 29713127 Free PMC article. Review. - TIFA Signaling in Gastric Epithelial Cells Initiates the cag Type 4 Secretion System-Dependent Innate Immune Response to Helicobacter pylori Infection.
Gall A, Gaudet RG, Gray-Owen SD, Salama NR. Gall A, et al. mBio. 2017 Aug 15;8(4):e01168-17. doi: 10.1128/mBio.01168-17. mBio. 2017. PMID: 28811347 Free PMC article. - Helicobacter pylori promotes the expression of Krüppel-like factor 5, a mediator of carcinogenesis, in vitro and in vivo.
Noto JM, Khizanishvili T, Chaturvedi R, Piazuelo MB, Romero-Gallo J, Delgado AG, Khurana SS, Sierra JC, Krishna US, Suarez G, Powell AE, Goldenring JR, Coffey RJ, Yang VW, Correa P, Mills JC, Wilson KT, Peek RM Jr. Noto JM, et al. PLoS One. 2013;8(1):e54344. doi: 10.1371/journal.pone.0054344. Epub 2013 Jan 23. PLoS One. 2013. PMID: 23372710 Free PMC article. - Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors.
Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, Wroblewski LE, Piazuelo MB, Correa P, Peek RM Jr. Franco AT, et al. Cancer Res. 2008 Jan 15;68(2):379-87. doi: 10.1158/0008-5472.CAN-07-0824. Cancer Res. 2008. PMID: 18199531 Free PMC article. - Role of NOD1 and ALPK1/TIFA Signalling in Innate Immunity Against Helicobacter pylori Infection.
Ying L, Ferrero RL. Ying L, et al. Curr Top Microbiol Immunol. 2019;421:159-177. doi: 10.1007/978-3-030-15138-6_7. Curr Top Microbiol Immunol. 2019. PMID: 31123889 Review.
Cited by
- Prognostic Implications of Pyroptosis-Related Gene Signatures in Lung Squamous Cell Carcinoma.
Li T, Liu H, Dong C, Lyu J. Li T, et al. Front Pharmacol. 2022 Jan 27;13:806995. doi: 10.3389/fphar.2022.806995. eCollection 2022. Front Pharmacol. 2022. PMID: 35153782 Free PMC article. - Helicobacter pylori elicits B7H3 expression on gastric epithelial cells: Implications in local T cell regulation and subset development during infection.
Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Lina TT, et al. Clin Oncol Res. 2019;2(5):10.31487/j.cor.2019.05.05. doi: 10.31487/j.cor.2019.05.05. Epub 2019 Oct 10. Clin Oncol Res. 2019. PMID: 31998864 Free PMC article. - Nucleotide-binding oligomerization domain 1 and Helicobacter pylori infection: A review.
Minaga K, Watanabe T, Kamata K, Asano N, Kudo M. Minaga K, et al. World J Gastroenterol. 2018 Apr 28;24(16):1725-1733. doi: 10.3748/wjg.v24.i16.1725. World J Gastroenterol. 2018. PMID: 29713127 Free PMC article. Review. - NOD1 mediates interleukin-18 processing in epithelial cells responding to Helicobacter pylori infection in mice.
Tran LS, Ying L, D'Costa K, Wray-McCann G, Kerr G, Le L, Allison CC, Ferrand J, Chaudhry H, Emery J, De Paoli A, Colon N, Creed S, Kaparakis-Liaskos M, Como J, Dowling JK, Johanesen PA, Kufer TA, Pedersen JS, Mansell A, Philpott DJ, Elgass KD, Abud HE, Nachbur U, Croker BA, Masters SL, Ferrero RL. Tran LS, et al. Nat Commun. 2023 Jun 26;14(1):3804. doi: 10.1038/s41467-023-39487-1. Nat Commun. 2023. PMID: 37365163 Free PMC article. - New Biology to New Treatment of Helicobacter pylori-Induced Gastric Cancer.
Peek RM Jr. Peek RM Jr. Dig Dis. 2016;34(5):510-6. doi: 10.1159/000445231. Epub 2016 Jun 22. Dig Dis. 2016. PMID: 27332886 Free PMC article.
References
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Rev Cancer. 2002;2:28–37. - PubMed
- Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to pylori. International Journal of Cancer Epub. 2014 May 29; - PubMed
- Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK053620/DK/NIDDK NIH HHS/United States
- P01 CA028842/CA/NCI NIH HHS/United States
- R01-DK58587/DK/NIDDK NIH HHS/United States
- R01 CA077955/CA/NCI NIH HHS/United States
- P01-CA116087/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30-DK058404/DK/NIDDK NIH HHS/United States
- P01 CA116087/CA/NCI NIH HHS/United States
- P01-CA028842/CA/NCI NIH HHS/United States
- I01 BX001453/BX/BLRD VA/United States
- R01 DK058587/DK/NIDDK NIH HHS/United States
- R01-CA77955/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical