Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis - PubMed (original) (raw)
Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis
M Alba Mañé Martínez et al. Mult Scler. 2015 Apr.
Abstract
Objective: To investigate glial and neuronal biomarkers in cerebrospinal fluid (CSF) samples from patients with relapsing-remitting multiple sclerosis (RRMS) and clinically isolated syndrome (CIS) suggestive of multiple sclerosis (MS), and to evaluate their ability to predict conversion from CIS to clinically definite MS (CDMS) and also disability progression in MS.
Methods: CSF levels of neurofilament light protein (NFL), t-tau, p-tau, glial fibrillary acidic protein (GFAP), S-100B, human chitinase 3-like 1 protein (YKL-40), monocyte chemoattractant protein-1 (MCP-1), α-sAPP and β-sAPP; and Aβ38, Aβ40 and Aβ42, were analyzed in 109 CIS patients and 192 RRMS patients. The mean follow-up time of these 301 patients was 11.7 ± 6.4 years.
Results: High levels of NFL were associated with early conversion from CIS to CDMS (hazard ratio (HR) with 95% confidence interval (CI): 2.69 (1.75 - 4.15); p < 0.0001). High levels of YKL-40 and GFAP were associated with earlier progression in the Expanded Disability Status Scale (EDSS), score 3: YKL-40 (HR (95% CI): 2.78 (1.48 - 5.23); p = 0.001) and GFAP (HR (95% CI): 1.83 (1.01 - 3.35); p = 0.04). High levels of YKL-40 were associated with earlier progression to EDSS 6 (HR (95% CI): 4.57 (1.01 - 20.83); p = 0.05).
Conclusions: CSF levels of NFL in CIS patients are an independent prognostic marker for conversion to CDMS. Whereas, CSF levels of YKL-40 and GFAP are independent prognostic markers for disability progression in MS.
Keywords: Biomarkers; cerebrospinal fluid; chitinase 3-like 1 protein; diagnostics; disability progression; glial fibrillary acidic protein; multiple sclerosis; neurofilament light protein; prognostic markers.
© The Author(s), 2015.
Conflict of interest statement
Conflict of interest: M Alba Mañé Martínez received research support from the Fundació Hospital Universitari de Tarragona Joan XXIII and Fundació Institut d’Investigació Biomèdica de Bellvitge (IDIBELL); and received research support, funding for travel and congress expenses from Biogen Idec, Teva Pharmaceutical Industries, Sanofi-Aventis, Merck Serono, Novartis and Bayer Schering Pharma.
Laura Bau received research support, funding for travel and congress expenses from Biogen Idec, Teva, Sanofi-Aventis, Merck Serono, Novartis and Bayer Schering pharmaceuticals.
Elisabet Matas received research support, funding for travel and congress expenses from Biogen Idec, Teva, Sanofi-Aventis, Merck Serono, Novartis and Bayer Schering pharmaceuticals.
Alvaro Cobo Calvo received research support, funding for travel and congress expenses from Biogen Idec, Teva, Sanofi-Aventis, Merck Serono, Novartis and Bayer Schering pharmaceuticals.
Kaj Blennow has served on the advisory board for Innogenetics of Belgium.
Lucia Romero-Pinel received research support, funding for travel and congress expenses from Biogen Idec, Teva, Sanofi-Aventis, Merck Serono, Novartis and Bayer Schering pharmaceuticals.
Sergio Martínez-Yélamos received honoraria compensation to participate in advisory boards, collaborations as a consultant and scientific communications from Biogen Idec, Teva, Sanofi-Aventis, Merck Serono, Novartis and Bayer Schering pharmaceuticals; and received research support, funding for travel and congress expenses from Biogen Idec, Teva, Sanofi-Aventis, Merck Serono, Novartis and Bayer Schering pharmaceuticals.
Ulf Andreasson, Bob Olsson and Henrik Zetterberg report no disclosures.
Figures
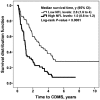
Figure 1.
NFL levels and conversion from CIS to CDMS. The Kaplan-Meier estimator was used to assess the time to develop CDMS. The median of CSF biomarker levels (NFL = 1150 ng/L) in the CIS group (n = 109) was established as the cut-off value, and used to classify CIS patients into two groups (high or low), respectively. The graph represents the survival distribution function in patients with high levels of NFL (n = 53) and low levels of NFL (n = 50). We display the median time to CDMS in years (with 95% CI). CDMS: Clinically-definite multiple sclerosis; CIS: clinically-isolated syndrome; MS: multiple sclerosis; NFL: neurofilament light protein; y: year/s
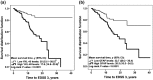
Figure 2.
YKL-40 and GFAP levels and disability progression in the relapsing forms of MS. We used a Kaplan-Meier estimator to assess the time to reach EDSS 3. The median of CSF biomarker levels (YKL-40 = 101 ng/mL and GFAP = 300 ng/L) in the relapsing-remitting forms of MS (CIS and RRMS) group (n = 301) was established as the cut-off value to classify MS patients into two groups (high or low), respectively. The mean survival time in years plus 95% CI are displayed. The time to reach EDSS 3 was significantly shorter in patients with high levels of (a) YKL-40 (n = 140) and (b) GFAP (n = 146), compared with patients with low levels of (a) YKL-40 (n = 140) and (b) GFAP (n = 155). aBecause YKL-40 was the last biomarker to be analyzed, 21 samples did not have enough volume left for the analysis. CIS: clinically-isolated syndrome; CSF: Cerebrospinal fluid; EDSS: Expanded Disability Status Scale; GFAP: glial fibrillary acidic protein; MS: multiple sclerosis; RRMS: relapsing–remitting MS; y: years; YKL-40: human chitinase 3-like 1 protein
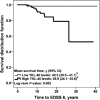
Figure 3.
YKL-40 levels and disability progression in the relapsing forms of MS. We used a Kaplan-Meier estimator to assess the time to reach EDSS 6. The median of CSF biomarker levels (YKL-40 = 101 ng/mL) in relapsing-remitting forms (CIS and RRMS) group (n = 301) was established as the cut-off value to classify MS patients into two groups (high or low), respectively. Mean survival time in years (y) with 95%CI are displayed. The time to reach EDSS 6 was significantly shorter in patients with high levels of YKL-40 (n = 140), compared with patients with low levels of YKL-40 (n = 140). aBecause YKL-40 was the last biomarker to be analyzed, 21 samples did not have enough volume left for the analysis. CIS: clinically-isolated syndrome; CSF: Cerebrospinal fluid; EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; RRMS: relapsing–remitting MS; y: years; YKL-40: human chitinase 3-like 1 protein
Similar articles
- Glial and neuronal markers in cerebrospinal fluid in different types of multiple sclerosis.
Mañé-Martínez MA, Olsson B, Bau L, Matas E, Cobo-Calvo Á, Andreasson U, Blennow K, Romero-Pinel L, Martínez-Yélamos S, Zetterberg H. Mañé-Martínez MA, et al. J Neuroimmunol. 2016 Oct 15;299:112-117. doi: 10.1016/j.jneuroim.2016.08.004. Epub 2016 Aug 24. J Neuroimmunol. 2016. PMID: 27725108 - Glial and neuroaxonal biomarkers in a multiple sclerosis (MS) cohort.
Kalatha T, Hatzifilippou E, Arnaoutoglou M, Balogiannis S, Koutsouraki E. Kalatha T, et al. Hell J Nucl Med. 2019 Sep-Dec;22 Suppl 2:113-121. Hell J Nucl Med. 2019. PMID: 31802051 - Cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain predict multiple sclerosis development and disability after optic neuritis.
Modvig S, Degn M, Roed H, Sørensen TL, Larsson HB, Langkilde AR, Frederiksen JL, Sellebjerg F. Modvig S, et al. Mult Scler. 2015 Dec;21(14):1761-70. doi: 10.1177/1352458515574148. Epub 2015 Feb 19. Mult Scler. 2015. PMID: 25698172 - Can CSF biomarkers predict future MS disease activity and severity?
Magliozzi R, Cross AH. Magliozzi R, et al. Mult Scler. 2020 Apr;26(5):582-590. doi: 10.1177/1352458519871818. Epub 2020 Jan 22. Mult Scler. 2020. PMID: 31965889 Review. - Neurofilaments as biomarkers in multiple sclerosis.
Teunissen CE, Khalil M. Teunissen CE, et al. Mult Scler. 2012 May;18(5):552-6. doi: 10.1177/1352458512443092. Epub 2012 Apr 4. Mult Scler. 2012. PMID: 22492131 Review.
Cited by
- Fluid biomarkers in multiple sclerosis: from current to future applications.
Di Filippo M, Gaetani L, Centonze D, Hegen H, Kuhle J, Teunissen CE, Tintoré M, Villar LM, Willemse EAJ, Zetterberg H, Parnetti L. Di Filippo M, et al. Lancet Reg Health Eur. 2024 Aug 22;44:101009. doi: 10.1016/j.lanepe.2024.101009. eCollection 2024 Sep. Lancet Reg Health Eur. 2024. PMID: 39444698 Free PMC article. Review. - Combined Cerebrospinal Fluid Neurofilament Light Chain Protein and Chitinase-3 Like-1 Levels in Defining Disease Course and Prognosis in Multiple Sclerosis.
Gil-Perotin S, Castillo-Villalba J, Cubas-Nuñez L, Gasque R, Hervas D, Gomez-Mateu J, Alcala C, Perez-Miralles F, Gascon F, Dominguez JA, Casanova B. Gil-Perotin S, et al. Front Neurol. 2019 Sep 23;10:1008. doi: 10.3389/fneur.2019.01008. eCollection 2019. Front Neurol. 2019. PMID: 31608004 Free PMC article. - The cerebrospinal fluid in multiple sclerosis: far beyond the bands.
Domingues RB, Fernandes GBP, Leite FBVM, Tilbery CP, Thomaz RB, Silva GS, Mangueira CLP, Soares CAS. Domingues RB, et al. Einstein (Sao Paulo). 2017 Jan-Mar;15(1):100-104. doi: 10.1590/S1679-45082017RW3706. Einstein (Sao Paulo). 2017. PMID: 28444098 Free PMC article. Review. - Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis.
Håkansson I, Tisell A, Cassel P, Blennow K, Zetterberg H, Lundberg P, Dahle C, Vrethem M, Ernerudh J. Håkansson I, et al. J Neuroinflammation. 2018 Jul 18;15(1):209. doi: 10.1186/s12974-018-1249-7. J Neuroinflammation. 2018. PMID: 30021640 Free PMC article. - Role of Chitinase 3-like 1 as a Biomarker in Multiple Sclerosis: A Systematic Review and Meta-analysis.
Floro S, Carandini T, Pietroboni AM, De Riz MA, Scarpini E, Galimberti D. Floro S, et al. Neurol Neuroimmunol Neuroinflamm. 2022 May 9;9(4):e1164. doi: 10.1212/NXI.0000000000001164. Print 2022 Jul. Neurol Neuroimmunol Neuroinflamm. 2022. PMID: 35534236 Free PMC article.
References
- Barkhof F, Calabresi PA, Miller DH, et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 2009; 5: 256–266. - PubMed
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol 1994; 4: 229–237. - PubMed
- Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338: 278–285. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous