The subventricular zone continues to generate corpus callosum and rostral migratory stream astroglia in normal adult mice - PubMed (original) (raw)
The subventricular zone continues to generate corpus callosum and rostral migratory stream astroglia in normal adult mice
Jiho Sohn et al. J Neurosci. 2015.
Abstract
Astrocytes are the most abundant cells in the CNS, and have many essential functions, including maintenance of blood-brain barrier integrity, and CNS water, ion, and glutamate homeostasis. Mammalian astrogliogenesis has generally been considered to be completed soon after birth, and to be reactivated in later life only under pathological circumstances. Here, by using genetic fate-mapping, we demonstrate that new corpus callosum astrocytes are continuously generated from nestin(+) subventricular zone (SVZ) neural progenitor cells (NPCs) in normal adult mice. These nestin fate-mapped corpus callosum astrocytes are uniformly postmitotic, express glutamate receptors, and form aquaporin-4(+) perivascular endfeet. The entry of new astrocytes from the SVZ into the corpus callosum appears to be balanced by astroglial apoptosis, because overall numbers of corpus callosum astrocytes remain constant during normal adulthood. Nestin fate-mapped astrocytes also flow anteriorly from the SVZ in association with the rostral migratory stream, but do not penetrate into the deeper layers of the olfactory bulb. Production of new astrocytes from nestin(+) NPCs is absent in the normal adult cortex, striatum, and spinal cord. Our study is the first to demonstrate ongoing SVZ astrogliogenesis in the normal adult mammalian forebrain.
Keywords: astroglia; corpus callosum; genetic fate-mapping; neural progenitor cells; rostral migratory stream; subventricular zone.
Copyright © 2015 the authors 0270-6474/15/353756-08$15.00/0.
Figures
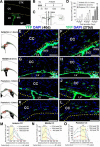
Figure 1.
Density and distribution analyses of nestin fate-mapped cells in the adult corpus callosum. A, Coronal brain section of 3-month-old NCER mice immunostained for EYFP 1 d after completion of a series of TM injections (i.p., 180 mg/kg body weight, daily for 4 consecutive days), showing EYFP+ cells are restricted to the SVZ. B, C, Experimental design: B, 3-month-old NCER mice received TM injections and were then killed at later time points (40, 120 or 270 d). C, Immunohistological analysis was done on coronal brain sections at 1.0 mm anterior, 0.0 mm and 1.0 mm posterior to the bregma (anterior, middle, and posterior corpus callosum, respectively). D, With increasing time post-TM injection, the density of EYFP+ cells increased in all 3 anterior–posterior regions of the corpus callosum (anterior CC: *p < 0.01 vs 40 d, **p < 0.005 vs 40 d, #p < 0.05 vs 120 d; middle CC: **p < 0.005 vs 40 d, ##p < 0.01 vs 120 d; posterior CC: **p < 0.005 vs 40 d, ##p < 0.01 vs 120 d). E–L, Images of EYFP+ cells in anterior (E, F), middle (G, H), and posterior (I–L) corpus callosum at 40 and 270 d after TM. The corpus callosum is demarcated by dotted lines. Rectangles in schematic drawings on the left mark the location of the images shown on the right. M–O, Mediolateral distributions of EYFP+ cells in anterior (M), middle (N), and posterior (O) corpus callosum. Note regional differences in mediolateral distributions of EYFP+ cells in different anterior–posterior regions of the corpus callosum. In the posterior corpus callosum (O), EYFP+ cells were more widely distributed whereas in more anterior segments (M, N) of the corpus callosum, EYFP+ cells were largely confined to the area close to the SVZ. Yellow areas in the graphs indicate mediolateral locations of the lateral ventricles. CC, Corpus callosum; CTX, cortex; LV, lateral ventricle; ST, striatum. Scale bars, 100 μm. Results are mean ± SEM (n = 3–4 brains).
Figure 2.
Lack of immature properties of nestin fate-mapped astrocytes in the adult corpus callosum. A, B, Images of the posterior corpus callosum at 270 d post-TM immunostained for EYFP and GFAP. C, The percentages of EYFP+ cells that were GFAP+ in the anterior, middle, and posterior corpus callosum, showing that the vast majority of EYFP+ cells were astrocytes. D, E, Images of the middle corpus callosum in close proximity to the SVZ at 120 d post-TM. Arrows indicate EYFP+/vimentin+/GFAP+ cells in the corpus callosum. Note that all EYFP+/vimentin+ cells were also GFAP+ and no EYFP+/vimentin+/GFAP− cells (i.e., presumably immature astrocytes) were detected in the corpus callosum. F, G, EYFP+/GFAP+ cells in the corpus callosum do not express nestin. H, Colocalization of nestin and PECAM (i.e., endothelial cells) expressions in the corpus callosum of adult wild-type mice. I, J, Orthogonal images of EYFP+/GFAP+/Ki67+ cell in the SVZ at 120 d post-TM, showing the presence of a fate-mapped mitotic astroglial cell (i.e., presumably type B cell). K, EYFP+/GFAP+ astrocytes in the corpus callosum do not proliferate. BV, Blood vessel; CC, corpus callosum; LV, lateral ventricle. Dotted lines in (A, B, D, E) demarcate the corpus callosum. Scale bars, 25 μm. Results are mean ± SEM (n = 3–4 brains).
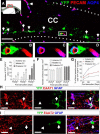
Figure 3.
Formation of perivascular endfeet and glutamate transporters by nestin fate-mapped astrocytes in the adult corpus callosum. A, Low-magnification image of the posterior corpus callosum at 270 d post-TM immunostained for EYFP, GFAP, and AQP4. A red rectangle in the schematic drawing shows the location of the low-magnification image. Arrows indicate the contacts between EYFP+/AQP4+ astrocytic endfeet and PECAM+ endothelial cells. Dotted lines demarcate the corpus callosum. B–D, Higher magnifications of the area marked by the white rectangle in A, showing EYFP+/AQP4+ astrocytic endfeet completely enwrapping a PECAM+ blood vessel. E, The density of EYFP+/AQP4+ perivascular astrocytes in the adult corpus callosum increased with increasing time after TM and toward more posterior regions of the corpus callosum (anterior CC: *p < 0.01 vs 40 d, **p < 0.005 vs 40 d, #p < 0.05 vs 120 d; middle CC: *p < 0.01 vs 40 d, **p < 0.005 vs 40 d, #p < 0.05 vs 120 d; posterior CC: **p < 0.005 vs 40 d, ##p < 0.01 vs 120 d). F, The density of PECAM+ blood vessels in different anterior–posterior regions of the adult corpus callosum. G, The percentages of PECAM+ blood vessels that were in contact with EYFP+/AQP4+ endfeet in anterior, middle, and posterior corpus callosum with increasing time after TM. Note that at 270 d after TM, 22.5% of total blood vessels in the posterior corpus callosum were associated with EYFP+/AQP4+ astrocytic endfeet. H, I, Both EYFP−/GFAP+ (arrowheads) and EYFP+/GFAP+ (arrows) astrocytes in the corpus callosum expressed excitatory amino acid transporters 1 (EAAT1; H) and EAAT2 (I), astrocyte-specific glutamate transporters. CC, Corpus callosum. Scale bars: A, 100 μm; H, I, 15 μm. Results are mean ± SEM (n = 3–4 brains).
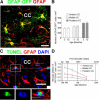
Figure 4.
Astrocyte turnover in the adult corpus callosum. A, Image of adult GFAP:GFP mouse corpus callosum immunostained for GFP and GFAP showing colocalization of GFAP:GFP and GFAP. B, Quantification of total numbers of GFAP:GFP+ astrocytes in the adult corpus callosum. C, Orthogonal view of a TUNEL+/GFAP+/DAPI+ cell in the adult corpus callosum. Insets below are the magnified images of the area outlined by the rectangle. Note convoluted nucleus with cavitation, a feature of early apoptosis (Johnson et al., 2000). D, Calculated net daily addition of EYFP+/GFAP+ astrocytes in the adult corpus callosum. CC: corpus callosum. Scale bars, 20 μm. Results are mean ± SEM (n = 3 brains in B, n = 3–4 brains in D).
Figure 5.
Nestin fate-mapping of astrocytes in the adult RMS. A, Schematic representation of a coronal forebrain section 1.0 mm anterior to bregma used for analysis (B–E) of the posterior RMS (pRMS). With increasing time post-TM, the density of nestin fate-mapped astrocytes (EYFP+/GFAP+) increased in the pRMS, as demonstrated by quantifications (B, C) and immunostaining (D, 7 d; E, 120 d post-TM). F–I, Anterior RMS (aRMS) visualized in sagittal (F, G) and coronal (H, I) sections. Note the robust stream of SVZ-derived EYFP+ cells in RMS at 120 d post-TM (G–I). Some of the EYFP+ cells were colabeled with DCX (H) or GFAP (G, I). Also, note the paucity of EYFP+ cells in the aRMS at 3 d post-TM (F), indicating that there were very few nestin+ NPCs in RMS, and validating the mapping strategy. J, K, RMS within the olfactory [RMS(ob)] at 120 d post-TM, showing some fate-mapped cells are astrocytes (EYFP+/GFAP+; K: magnified image of the boxed area in J). L, EYFP+ astrocytes are absent in the GCL at 120 d post-TM. Insets in D, E, and G are magnified images of the boxed areas. Arrowheads in G, I, and K indicate fate-mapped astrocytes (EYFP+/GFAP+). The RMS is highlighted by dotted lines. CC, Corpus callosum; LV, lateral ventricle. Scale bars: D–G, J, 100 μm; H, I, L, 50 μm. *p < 0.01 versus 7 d, #p < 0.05 versus 40 d in B; *p < 0.01 versus 7 d, **p < 0.001 versus 7 d, #p < 0.01 versus 40 d in C. Results are mean ± SEM (n = 3–4 brains).
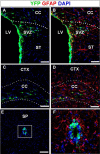
Figure 6.
Absence of NSC-derived astroglial production in the forebrain gray matter and the spinal cord in normal adults. A–D, Low-magnification images of coronal brain sections at 270 d post-TM. EYFP+/GFAP+ cells migrated into the corpus callosum in both close proximity to the SVZ (A, B) and distal to the SVZ (C, D), but did not enter the striatum (A, B) or cortex (C, D). E, The absence of EYFP+ cells outside the central cannel at 270 d post-TM. F, Higher-magnification image of the area outlined by a rectangle in E, showing the lack of NSC-derived astrogliogenesis in the adult spinal cord. CC, Corpus callosum; CTX, cortex; LV, lateral ventricle; SP, spinal cord; ST, striatum. Scale bars, 100 μm.
Similar articles
- Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum.
Guglielmetti C, Praet J, Rangarajan JR, Vreys R, De Vocht N, Maes F, Verhoye M, Ponsaerts P, Van der Linden A. Guglielmetti C, et al. Neuroimage. 2014 Feb 1;86:99-110. doi: 10.1016/j.neuroimage.2013.07.080. Epub 2013 Aug 7. Neuroimage. 2014. PMID: 23933305 - A Distinct Population of Microglia Supports Adult Neurogenesis in the Subventricular Zone.
Ribeiro Xavier AL, Kress BT, Goldman SA, Lacerda de Menezes JR, Nedergaard M. Ribeiro Xavier AL, et al. J Neurosci. 2015 Aug 26;35(34):11848-61. doi: 10.1523/JNEUROSCI.1217-15.2015. J Neurosci. 2015. PMID: 26311768 Free PMC article. - Neural-specific inactivation of ShcA functions results in anatomical disorganization of subventricular zone neural stem cell niche in the adult brain.
Ponti G, Reitano E, Aimar P, Cattaneo E, Conti L, Bonfanti L. Ponti G, et al. Neuroscience. 2010 Jun 16;168(1):314-22. doi: 10.1016/j.neuroscience.2010.03.008. Epub 2010 Mar 10. Neuroscience. 2010. PMID: 20226234 - The multifaceted subventricular zone astrocyte: From a metabolic and pro-neurogenic role to acting as a neural stem cell.
Platel JC, Bordey A. Platel JC, et al. Neuroscience. 2016 May 26;323:20-8. doi: 10.1016/j.neuroscience.2015.10.053. Epub 2015 Nov 3. Neuroscience. 2016. PMID: 26546469 Free PMC article. Review. - Overlapping migratory mechanisms between neural progenitor cells and brain tumor stem cells.
Zarco N, Norton E, Quiñones-Hinojosa A, Guerrero-Cázares H. Zarco N, et al. Cell Mol Life Sci. 2019 Sep;76(18):3553-3570. doi: 10.1007/s00018-019-03149-7. Epub 2019 May 17. Cell Mol Life Sci. 2019. PMID: 31101934 Free PMC article. Review.
Cited by
- Aberrations of Genomic Imprinting in Glioblastoma Formation.
Lozano-Ureña A, Jiménez-Villalba E, Pinedo-Serrano A, Jordán-Pla A, Kirstein M, Ferrón SR. Lozano-Ureña A, et al. Front Oncol. 2021 Mar 12;11:630482. doi: 10.3389/fonc.2021.630482. eCollection 2021. Front Oncol. 2021. PMID: 33777782 Free PMC article. Review. - Single-Cell Analysis of Regional Differences in Adult V-SVZ Neural Stem Cell Lineages.
Mizrak D, Levitin HM, Delgado AC, Crotet V, Yuan J, Chaker Z, Silva-Vargas V, Sims PA, Doetsch F. Mizrak D, et al. Cell Rep. 2019 Jan 8;26(2):394-406.e5. doi: 10.1016/j.celrep.2018.12.044. Cell Rep. 2019. PMID: 30625322 Free PMC article. - Intravital Imaging of the Murine Subventricular Zone with Three Photon Microscopy.
Sun B, Wang M, Hoerder-Suabedissen A, Xu C, Packer AM, Szele FG. Sun B, et al. Cereb Cortex. 2022 Jul 12;32(14):3057-3067. doi: 10.1093/cercor/bhab400. Cereb Cortex. 2022. PMID: 35029646 Free PMC article. - A simple solution for antibody signal enhancement in immunofluorescence and triple immunogold assays.
Rosas-Arellano A, Villalobos-González JB, Palma-Tirado L, Beltrán FA, Cárabez-Trejo A, Missirlis F, Castro MA. Rosas-Arellano A, et al. Histochem Cell Biol. 2016 Oct;146(4):421-30. doi: 10.1007/s00418-016-1447-2. Epub 2016 May 17. Histochem Cell Biol. 2016. PMID: 27188756 - "Till Death Do Us Part": A Potential Irreversible Link Between Aberrant Cell Cycle Control and Neurodegeneration in the Adult Olfactory Bulb.
Omais S, Jaafar C, Ghanem N. Omais S, et al. Front Neurosci. 2018 Mar 9;12:144. doi: 10.3389/fnins.2018.00144. eCollection 2018. Front Neurosci. 2018. PMID: 29593485 Free PMC article. Review.