Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer's disease - PubMed (original) (raw)
Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer's disease
Dan Xia et al. Neuron. 2015.
Abstract
Presenilins play essential roles in memory formation, synaptic function, and neuronal survival. Mutations in the Presenilin-1 (PSEN1) gene are the major cause of familial Alzheimer's disease (FAD). How PSEN1 mutations cause FAD is unclear, and pathogenic mechanisms based on gain or loss of function have been proposed. Here, we generated Psen1 knockin (KI) mice carrying the FAD mutation L435F or C410Y. Remarkably, KI mice homozygous for either mutation recapitulate the phenotypes of Psen1(-/-) mice. Neither mutation altered Psen1 mRNA expression, but both abolished γ-secretase activity. Heterozygosity for the KI mutation decreased production of Aβ40 and Aβ42, increased the Aβ42/Aβ40 ratio, and exacerbated Aβ deposition. Furthermore, the L435F mutation impairs hippocampal synaptic plasticity and memory and causes age-dependent neurodegeneration in the aging cerebral cortex. Collectively, our findings reveal that FAD mutations can cause complete loss of Presenilin-1 function in vivo, suggesting that clinical PSEN mutations produce FAD through a loss-of-function mechanism.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
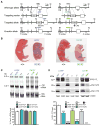
Figure 1. L435F and C410Y KI/KI mice exhibit phenotypes indistinguishable from _Psen1_−/− mice
(A) Targeting strategy for the generation of L435F and C410Y KI mice. Boxes represent Psen1 exons 8–12, and the locations of the 5′ and 3′ external probes are shown. The L435F mutation is located in exon 12 and C410Y mutation is located in exon 11. Ba and Ap denote _Bam_HI and _Apa_I restriction sites, respectively. (B) Newborn L435F and C410Y KI/KI pups resemble _Psen1_−/− pups with shortened tail and body axis. (C) Northern analysis of brain total RNA at P0 shows normal Psen1 mRNA size and level in L435F and C410Y KI/KI and KI/+ mice (n≥5). (D) Western analysis of brain lysates at P0 shows residual amounts of PS1 NTFs and CTFs in L435F and C410Y KI/KI brains and drastic accumulation of PS1 holoprotein in L435F and C410Y KI/KI and KI/+ brains in a KI gene dosage-dependent manner, whereas these PS1 species are undetectable in _Psen1_−/− brains (n≥4). Data are represented as mean ± SEM. ***p < 0.001. See alsoFigure S1 and Table S1.
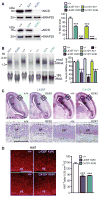
Figure 2. Impaired neurogenesis and Notch signaling in L435F and C410Y KI/KI mice
(A) _In vitro γ-_secretase assay using CHAPSO-solubilized E18.5 brain fractions and recombinant Notch substrate N102-FmH followed by Western analysis reveals that NICD production is reduced in L435F KI/+ (n=3) and C410Y KI/+ (n=4) mice, and abolished in L435F KI/KI (n=3) and C410Y KI/KI (n=3) mice, compared to Psen1+/+ controls (n=6). SNAP25 is used as internal loading control for membrane fractions. (B) Northern analysis of Hes5 expression. Levels of Hes5 mRNA are markedly reduced in L435F KI/KI (n=9), C410Y KI/KI (n=3), Psen1−/− (n=3) brains relative to Psen1+/+ controls (n=7). (C) Hematoxylin/eosin staining of comparable brain sections from L435F KI/KI, C410Y KI/KI and littermate controls at E16.5 shows that the ventricular zone (VZ) and the developing cortical plate (CP) are thinner in L435F KI/KI and C410Y KI/KI mice. The bottom panels show higher power views of the boxed areas in the developing CP. Dashed lines show the boundaries of the CP. Scale bar: 0.25mm. (D) The number of proliferating neural progenitor cells in the VZ labeled by Ki67 immunoreactivity is reduced in L435F KI/KI and C410Y KI/KI brains at E16.5 (n=4 for each genotype). Scale bar: 0.25mm. Data are represented as mean ± SEM. ***p < 0.001.
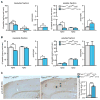
Figure 3. Abolished Aβ production in L435F KI/KI mice and reduced Aβ40 and Aβ42 production in L435F KI/+ mice
(A) APP CTFs accumulate (>10-fold) in L435F KI/KI and Psen1−/− brains relative to littermate controls at E18.5 (n≥6). (B) ELISA measurements of Aβ40 and Aβ42 following in vitro γ-secretase assay. In vitro γ-secretase assay reveals that de novo generation of Aβ40 and Aβ42 in L435F KI/+ brains at E18.5 (n=4) is reduced by 46% and 43%, respectively, compared to Psen1+/+ brains (n=5), and they are undetectable in L435F KI/KI brains (n=3). (C) ELISA measurements of endogenous Aβ40 and Aβ42 from brain homogenates at E18.5. Levels of stead-state endogenous Aβ40 and Aβ42 are similar between L435F KI/+ and Psen1+/+ brains, and they are undetectable in L435F KI/KI brains (n=4 for each genotype). (D) ELISA measurements of Aβ40 and Aβ42 following in vitro γ-secretase assay in 3-month old mice. De novo generation of Aβ40 and Aβ42 in L435F KI/+ cortical homogenates is reduced by 47% and 43%, respectively, compared to Psen1+/+ cortices (n=6 for each genotype). (E) ELISA measurements of stead-state endogenous Aβ40 and Aβ42 in both insoluble and soluble fractions from cortical samples at 3 months of age. In insoluble fractions, levels of endogenous Aβ40 and Aβ42 are reduced in L435F KI/+ cortices (n=10) relative to controls (n=7), and the Aβ42/Aβ40 ratio is increased. In soluble fractions, levels of endogenous Aβ40 and Aβ42 are not significantly different between Psen1L435F/+ and Psen1+/+ cortices, but the Aβ42/Aβ40 ratio is significantly increased in Psen1L435F/+ cortices. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. See alsoFigure S2 and Table S2.
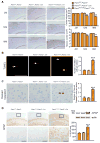
Figure 4. Reduced human Aβ accumulation but accelerated amyloid deposition in L435F KI/+ mice expressing a human mutant APP transgene
(A) ELISA measurements of steady-state human Aβ40 and Aβ42 in insoluble and soluble fractions from Psen1L435F/+; APPWT (n=4) and controls (n=4) at 9 months of age. In Psen1L435F/+; APPWT mice, human Aβ40 is reduced by ~26% in insoluble fractions and ~62% in soluble fractions, whereas human Aβ42 is reduced by ~19% in insoluble fractions but not significant changed in soluble fractions. Note that levels of Aβ40 and Aβ42 are much higher in insoluble fractions than in soluble fractions (>20-fold for Aβ40, >10-fold for Aβ42). The Aβ42/Aβ40 ratio is significantly enhanced in soluble and insoluble fractions. (B) ELISA measurements of steady-state human Aβ40 and Aβ42 in insoluble and soluble fractions of cortical homogenates from Psen1+/+; APPMT (n=4) and Psen1L435F/+; APPMT (n=4) at 2 months of age. Levels of human Aβ40 are significantly reduced in insoluble fractions but not in soluble fractions, whereas levels of human Aβ42 are not significantly altered. Note that levels of Aβ40 are drastically higher in insoluble fractions than in soluble fractions (>100-fold). The Aβ42/Aβ40 ratio is significantly increased in insoluble fractions. (C) Aβ immunostaining with an Aβ antibody (R1282) reveals significantly increased plaque deposition in the cerebral cortex of Psen1L435F/+; APPMT mice compared to Psen1+/+; APPMT mice at 9 months of age. Scale bar: 0.25 mm. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. See alsoFigure S3 and Table S2.
Figure 5. Memory impairment caused by the L435F mutation
(A) Psen1L435F/+; _Psen2_−/− (KI/+; −/−) mice (n=14) showed significantly higher escape latencies than Psen1+/+; _Psen2_−/− (+/+; −/−) littermates (n=15) during the 14-day training period (F1, 27 = 8.06; p<0.01) in the Morris water maze test. (B) _Psen1L435F/+_; _Psen2_−/− mice show significantly reduced target quadrant occupancy in the day 7 probe test, but both genotypic groups show similar target quadrant occupancy in the day 13 probe test. _Psen1L435F/+_; _Psen2_−/− mice display significantly reduced quadrant occupancy in the partial-cue probe test on day 14. T, target quadrant; AL, adjacent left quadrant; AR, adjacent right quadrant; OP, opposite quadrant. (C) Naïve _Psen1L435F/+_; _Psen2_−/− mice (n=10) and littermate controls (n=9) were subjected to 8 days of training to locate the three baited arms (indicated by red *) following fasting in the radial arm maze test. (D) _Psen1L435F/+_; _Psen2_−/− and control mice require similar amounts of time to complete the food search (F1, 17 = 1.18, p > 0.05) during the 8-day radial arm maze training. (E) Psen1L435F/+; _Psen2_−/− mice display significantly more reference errors (F1, 17 = 4.59, p < 0.05) during the 8-day training period. (F) Psen1L435F/+; _Psen2_−/− mice show significantly higher percentages of 45 degree turns (F1, 17 = 13.63, p < 0.001) during the 8-day training period. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. See alsoFigure S4.
Figure 6. Synaptic dysfunction caused by the L435F mutation
(A) Normal basal synaptic transmission in Psen1L435F/+; _Psen2_−/− mice. The synaptic input-output relationship was obtained by plotting the fiber volley amplitude against the initial slope of the evoked fEPSP. Each point represents data averaged across all slices for a narrow bin of FV amplitude. The lines represent the best linear regression fit. (B) Reduced paired-pulse facilitation (PPF) in Psen1L435F/+; _Psen2_−/− mice. The graph depicts the paired-pulse response ratio obtained at different inter-stimulus intervals (in ms). Scale bar: 1 mV, 50 ms. (C) Synaptic facilitation elicited by stimulus trains of the indicated frequencies in Psen1L435F/+; _Psen2_−/− and littermate control mice. Frequency facilitation is significantly decreased in the 5–20 Hz range in Psen1L435F/+; _Psen2_−/− mice. Scale bar: 1 Hz: 1 mV, 500 ms; 20 Hz: 1 mV, 50 ms. FP, field potential. (D) Pairing-induced LTP at the Schaffer collateral-CA1 synapses in slices from Psen1L435F/+; _Psen2_−/− mice and littermate controls. Representative traces before (thin) and after (thick) LTP induction are shown. Insets show the average of 15 EPSCs recorded before and 45–50 min after the induction. Scale bar: 20 ms, 50 pA. (E) LTP induced at the recurrent commissural/associational (C/A)-CA3 synapses in slices from Psen1L435F/+; _Psen2_−/− mice and littermate controls. LTP was induced with 3 trains of high frequency stimulation (HFS). Representative traces before (thin) and after (thick) LTP induction are shown. Superimposed traces are averages of four consecutive responses 1 min before and 50 min after HFS. The lack of response to DCG IV, which selectively inhibits mossy fiber LTP, confirms that the responses are obtained from (C/A)-CA3 synapses. Scale bar: 10 ms, 0.5 mV. (F) Increased synaptic depression at (C/A)-CA3 synapses during LTP-inducing stimulation in Psen1L435F/+; _Psen2_−/− mice. Insets show representative fEPSP traces obtained during single stimulation train. Scale bar, 10 ms, 2 mV. The values in parentheses indicate the number of neurons or slices (left) and the number of mice (right) used in each experiment. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. See alsoFigure S5.
Figure 7. The L435F mutation causes age-dependent neurodegeneration
(A) Comparison of brain morphology between Psen1F/L435F; Psen2_−/−; Cre_ mice and littermate controls at 2, 12 and 18 months of age. Left: Images of Nissl stained comparable sagittal brain sections. Right: Cortical volume and neuron number obtained by stereological quantification following Nissl staining and NeuN immnostaining, respectively. Cortical volume and neuron number are normal at 2 months of age but significantly reduced at 12 (~22%) and 18 (~32%) months of age in Psen1F/L435F; Psen2_−/−; Cre_ mice relative to controls (n≥3 for each genotype at each age). Scale bar: 0.1 mm. (B) More TUNEL-positive cells are observed in the cerebral cortex of Psen1F/L435F; Psen2_−/−; Cre_ mice (n=3), compared to control mice (n=4) at 18 months of age. Scale bar: 0.1 mm. (C) More active caspase3-positive cells are detected in the cerebral cortex of Psen1F/L435F; Psen2_−/−; Cre_ mice (n=3), relative to control mice (n=4) at 18 months of age. Scale bar: 0.1 mm. (D) Increased GFAP immunoreactivity in the cortex of Psen1F/L435F; Psen2_−/−; Cre_ mice at 12 months of age indicates astrogliosis. Bottom images show higher magnification views of the boxed area in the cortex. Western analysis also shows increased levels of GFAP in the cortex of Psen1F/L435F; Psen2_−/−; Cre_ mice (KI) compared to littermate control Psen1F/F; Psen2_−/− and Psen1F/+; Psen2_−/−; Cre mice (n=8 for each genotype). Data are represented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001. Scale bar: 0.1mm. See alsoFigure S6.
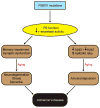
Figure 8. A schematic model for FAD
Pathogenic PSEN1 mutations cause loss of PS1 function and γ-secretase activity, leading to AD-relevant functional and neuropathological changes, including memory impairment, synaptic dysfunction, age-dependent neurodegeneration, gliosis and dementia. In parallel, the loss of PS function produced by pathogenic PSEN1 mutations also reduces Aβ production and increases the Aβ42/Aβ40 ratio due to the greater reduction of Aβ40 production than Aβ42, thereby promoting amyloid plaque deposition. Thus, PSEN mutations produce the full spectrum of AD phenotypes through a loss-of-function mechanism.
Comment in
- More than a FAD: the in vivo effects of disease-linked presenilin-1 mutations.
Zahs KR, Ashe KH. Zahs KR, et al. Neuron. 2015 Mar 4;85(5):893-5. doi: 10.1016/j.neuron.2015.02.021. Neuron. 2015. PMID: 25741717 - Familial Alzheimer's Disease Mutations in Presenilin Generate Amyloidogenic Aβ Peptide Seeds.
Veugelen S, Saito T, Saido TC, Chávez-Gutiérrez L, De Strooper B. Veugelen S, et al. Neuron. 2016 Apr 20;90(2):410-6. doi: 10.1016/j.neuron.2016.03.010. Neuron. 2016. PMID: 27100199 - Loss of Aβ43 Production Caused by Presenilin-1 Mutations in the Knockin Mouse Brain.
Xia D, Kelleher RJ 3rd, Shen J. Xia D, et al. Neuron. 2016 Apr 20;90(2):417-22. doi: 10.1016/j.neuron.2016.03.009. Neuron. 2016. PMID: 27100200 Free PMC article.
Similar articles
- Cortical neurodegeneration caused by Psen1 mutations is independent of Aβ.
Yan K, Zhang C, Kang J, Montenegro P, Shen J. Yan K, et al. Proc Natl Acad Sci U S A. 2024 Aug 20;121(34):e2409343121. doi: 10.1073/pnas.2409343121. Epub 2024 Aug 13. Proc Natl Acad Sci U S A. 2024. PMID: 39136994 Free PMC article. - Loss of Aβ43 Production Caused by Presenilin-1 Mutations in the Knockin Mouse Brain.
Xia D, Kelleher RJ 3rd, Shen J. Xia D, et al. Neuron. 2016 Apr 20;90(2):417-22. doi: 10.1016/j.neuron.2016.03.009. Neuron. 2016. PMID: 27100200 Free PMC article. - Human Presenilin-1 delivered by AAV9 rescues impaired γ-secretase activity, memory deficits, and neurodegeneration in Psen mutant mice.
Montenegro P, Chen P, Kang J, Lee SH, Leone S, Shen J. Montenegro P, et al. Proc Natl Acad Sci U S A. 2023 Oct 17;120(42):e2306714120. doi: 10.1073/pnas.2306714120. Epub 2023 Oct 10. Proc Natl Acad Sci U S A. 2023. PMID: 37816062 Free PMC article. - Evidence For and Against a Pathogenic Role of Reduced γ-Secretase Activity in Familial Alzheimer's Disease.
Jayne T, Newman M, Verdile G, Sutherland G, Münch G, Musgrave I, Moussavi Nik SH, Lardelli M. Jayne T, et al. J Alzheimers Dis. 2016 Apr 4;52(3):781-99. doi: 10.3233/JAD-151186. J Alzheimers Dis. 2016. PMID: 27060961 Review. - Exploring the Role of PSEN Mutations in the Pathogenesis of Alzheimer's Disease.
Kabir MT, Uddin MS, Setu JR, Ashraf GM, Bin-Jumah MN, Abdel-Daim MM. Kabir MT, et al. Neurotox Res. 2020 Dec;38(4):833-849. doi: 10.1007/s12640-020-00232-x. Epub 2020 Jun 18. Neurotox Res. 2020. PMID: 32556937 Review.
Cited by
- Clinical relevance of biomarkers, new therapeutic approaches, and role of post-translational modifications in the pathogenesis of Alzheimer's disease.
Mumtaz I, Ayaz MO, Khan MS, Manzoor U, Ganayee MA, Bhat AQ, Dar GH, Alghamdi BS, Hashem AM, Dar MJ, Ashraf GM, Maqbool T. Mumtaz I, et al. Front Aging Neurosci. 2022 Sep 7;14:977411. doi: 10.3389/fnagi.2022.977411. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36158539 Free PMC article. Review. - Spatiotemporal patterns of gliosis and neuroinflammation in presenilin 1/2 conditional double knockout mice.
Peng W, Xie Y, Liao C, Bai Y, Wang H, Li C. Peng W, et al. Front Aging Neurosci. 2022 Sep 14;14:966153. doi: 10.3389/fnagi.2022.966153. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36185485 Free PMC article. - The vexing complexity of the amyloidogenic pathway.
Castro MA, Hadziselimovic A, Sanders CR. Castro MA, et al. Protein Sci. 2019 Jul;28(7):1177-1193. doi: 10.1002/pro.3606. Epub 2019 Apr 11. Protein Sci. 2019. PMID: 30897251 Free PMC article. Review. - The genes associated with early-onset Alzheimer's disease.
Dai MH, Zheng H, Zeng LD, Zhang Y. Dai MH, et al. Oncotarget. 2017 Dec 15;9(19):15132-15143. doi: 10.18632/oncotarget.23738. eCollection 2018 Mar 13. Oncotarget. 2017. PMID: 29599933 Free PMC article. Review. - Network abnormalities and interneuron dysfunction in Alzheimer disease.
Palop JJ, Mucke L. Palop JJ, et al. Nat Rev Neurosci. 2016 Dec;17(12):777-792. doi: 10.1038/nrn.2016.141. Epub 2016 Nov 10. Nat Rev Neurosci. 2016. PMID: 27829687 Free PMC article. Review.
References
- Beglopoulos V, Sun X, Saura CA, Lemere CA, Kim RD, Shen J. Reduced beta-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice. J Biol Chem. 2004;279:46907–46914. - PubMed
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
- Campion D, Flaman JM, Brice A, Hannequin D, Dubois B, Martin C, Moreau V, Charbonnier F, Didierjean O, Tardieu S, et al. Mutations of the presenilin I gene in families with early-onset Alzheimer’s disease. Hum Mol Genet. 1995;4:2373–2377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS075346/NS/NINDS NIH HHS/United States
- R01NS041783/NS/NINDS NIH HHS/United States
- R01NS075346/NS/NINDS NIH HHS/United States
- R01 NS042818/NS/NINDS NIH HHS/United States
- R01NS042818/NS/NINDS NIH HHS/United States
- RF1 NS041783/NS/NINDS NIH HHS/United States
- R01 NS041783/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases