Normal and leukemic stem cell niches: insights and therapeutic opportunities - PubMed (original) (raw)
Review
Normal and leukemic stem cell niches: insights and therapeutic opportunities
Koen Schepers et al. Cell Stem Cell. 2015.
Abstract
Hematopoietic stem cells (HSCs) rely on instructive cues from the bone marrow (BM) niche to maintain their quiescence and adapt blood production to the organism's needs. Alterations in the BM niche are commonly observed in blood malignancies and directly contribute to the aberrant function of disease-initiating leukemic stem cells (LSCs). Here, we review recent insights into the cellular and molecular determinants of the normal HSC niche and describe how genetic changes in stromal cells and leukemia-induced BM niche remodeling contribute to blood malignancies. Moreover, we discuss how these findings can be applied to non-cell-autonomous therapies targeting the LSC niche.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Figure 1. Organization of the HSC niche
A) Overall anatomy of the marrow cavity depicting the sympathetic innervation and the vasculature, and highlighting the interconnection between arteriole and sinusoid blood vessels. Each of these regions (dotted box) is enriched for a particular subset of perivascular MSCs, which controls a different HSC functional state. Quiescent HSCs are G0 dormant cells. Active HSCs are cells that have just exited quiescence or are already actively cycling or migrating. B) Blow up of the essential (black) and accessory (grey) HSC niche cells with their respective secreted and/or cell-bound factors (color-coded) that regulate HSC functional states. Doted circles group cells with either similar origin (i.e., perivascular MSC subsets and differentiating OBCs) or similar function (i.e., specialized macrophages (Mac) and SNS components). Black arrows highlight MSC progeny that are differentiating into bone-lining OBs and forming the OBC compartment. Grey arrows indicate the long-range, indirect effects of several accessory HSC niche cells. CAR: CXCL12bright MSCs; E-Sel: E-selectin; LEP-R: NG2−LEP-R+Nesbright MSCs; NG2: NG2+LEP-R−Nesdim MSCs; nmSC: non-myelinating Schwann cells; NorE: norepinephrine; OCL: osteoclasts; OPr: osteoprogenitors; OsM: osteomacs.
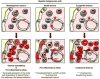
Figure 2. Models of disease initiation
The majority of myeloid malignancies are caused by genetic lesions (stars) occurring in hematopoietic cells, which lead to BM niche remodeling and formation of LSCs with deranged perception of the microenvironment. Genetic lesions can also occur in stromal cells and lead to myeloid malignancies with predisposition to secondary mutations in hematopoietic cells. These two modes of disease initiation are not mutually exclusive as they share several common mechanisms and self-reinforcing loops including deregulated Notch/Wnt signaling and increased production of pro-inflammatory cytokines. Congenital lesions present in both hematopoietic and stromal cells are also observed in myeloid malignancies, and likely synergize as well as predispose for additional transforming lesions and more aggressive diseases. Arrows indicate the directionality of these events.
Figure 3. Self-reinforcing malignant BM niches
Mechanisms identified in the indicated murine models, which create a self-reinforcing malignant BM niche promoting disease maintenance at the expense of normal hematopoiesis. Several key features have emerged as common themes from these analyses and include (1) altered MSC growth and differentiation with eventual fibrosis, (2) elaboration of pro-inflammatory signals, and (3) decreased production of HSC-supportive factors by stromal cells.
References
- Abdel-Wahab OI, Levine RL. Primary myelofibrosis: update on definition, pathogenesis, and treatment. Annu Rev Med. 2009;60:23–245. - PubMed
- Aizawa S, Nakano M, Iwase O, Yaguchi M, Hiramoto M, Hoshi H, Nabeshima R, Shima D, Handa H, Toyama K. Bone marrow stroma from refractory anemia of myelodysplastic syndrome is defective in its ability to support normal CD34-positive cell proliferation and differentiation in vitro. Leukemia Research. 1999;23:239–246. - PubMed
- Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. - PubMed
- Arranz L, Sánchez-Aguilera A, Martín-Pérez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntión S, Tzeng YS, Lai DM, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. - PubMed
- Asada N, Katayama Y, Sato M, Minagawa K, Wakahashi K, Kawano H, Kawano Y, Sada A, Ikeda K, Matsui T, et al. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell. 2013;12:737–747. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical