Tenogenic Induction of Human MSCs by Anisotropically Aligned Collagen Biotextiles - PubMed (original) (raw)
Tenogenic Induction of Human MSCs by Anisotropically Aligned Collagen Biotextiles
Mousa Younesi et al. Adv Funct Mater. 2014.
Abstract
A novel biofabrication modality, electrophoretic compaction with macromolecular alignment, was utilized to make collagen threads that mimic the native tendon's structure and mechanical properties. A device with kinematic electrodes was designed to fabricate collagen threads in continuous length. For the first time, a 3D-biotextile was woven purely from collagen. Mechanical properties and load-displacement behavior of the biotextile mimicked those of the native tendon while presenting a porosity of 80%. The open pore network facilitated cell seeding across the continuum of the bioscaffold. Mesenchymal stem cells (MSCs) seeded in the woven scaffold underwent tenogenic differentiation in the absence of growth factors and synthesized a matrix that was positive for tenomodulin, COMP and type I collagen. Up-regulation of tenomodulin, a tendon specific marker, was 11.6 ± 3.5 fold, COMP was up-regulated 16.7 ± 5.5 fold, and Col I was up-regulated 6.9 ± 2.7 fold greater on ELAC threads when compared to randomly oriented collagen gels. These results demonstrate that a bioscaffold woven by using collagen threads with densely compacted and anisotropically aligned substrate texture stimulates tenogenesis topographically, rendering the electrochemically aligned collagen as a promising candidate for functional repair of tendons and ligaments.
Keywords: Tissue engineering; cell alignment; cell differentiation; substrate stiffness anisotropy.
Figures
Figure 1
Schematic of the rotating electrode electrochemical alignment device (a). The main parts of the device are: power supply for providing voltage for the electrochemical cell, the syringe pump, rotating electrodes wheel and collection spool. Compensated polarized image in the top left inset demonstrates the collagen molecules to be aligned parallel to the longer axis of the thread as manifested by the blue color. Closely packed and aligned topography of the fiber surface is evident from the electron microscopy image. (b) Collagen fiber made by REEAD, (c) yarn made by twisting three collagen threads, (d) pin-setup for weaving the collagen scaffold, (e) the resulting woven collagen scaffold, (f) and two scaffolds to demonstrate the consistency of fabrication.
Figure 2
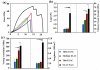
Mechanical assessment of ELAC threads with three different thicknesses and ELAC yarn. (a) Typical stress-strain curves of ELAC threads and ELAC yarn, (b) Failure load and strength of ELAC yarn is greater than individual ELAC threads, (c) toughness and Young’s Modulus of ELAC yarn was greater than those of ELAC threads. The horizontal line indicates significant difference (p<0.05).
Figure 3
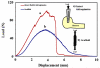
Tensile behavior of the scaffold (a) converges to that of similarly sized rabbit infraspinatus tendon (b).
Figure 4
Histological sections of the ELAC bioscaffold stained with H&E at day 3 (a) and at day 35 (b). (a) High magnification image shows uniform distribution and penetration of cells within the ELAC bioscaffold at day 3. Cells have round morphology at this point in time. (b) Spindle shaped elongated cell morphology was observed around the ELAC threads by day 35. (c) A macrograph of cell-seeded scaffold where the cellular F-actin cytoskeleton is labeled. Cells have profusely covered the entire scaffold with elongated morphology.
Figure 5
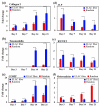
Topographical cues from the scaffold guide cells toward expression of the tendon specific/related proteins. RT-PCR results show significant up-regulation of Collagen I (a), tenomodulin (b), and COMP (c) on ELAC threads compared to random collagen samples at every time point. However, the expression of bone-related markers ALP (d) and RUNX2 (e) decreased over time. Osteocalcin expression was greater on random gel in comparison to ELAC threads. The expression of osteocalcin increased with time on random gels and did not change appreciably on ELAC threads. All bar graphs are normalized by expression at day 3 random group. The horizontal lines indicate significant differences (p<0.05) between expressions of the markers on random collagen samples vs. ELAC threads.
Figure 6
Immunohistochemistry of ELAC bioscaffolds for tendon-specific and tendon-related markers (Day 35). Type I collagen deposition was observed within the pores of the ELAC bioscaffold (a). Sections were positive for the tendon specific marker tenomodulin (b), and the tendon-related marker COMP (c).
Figure 7
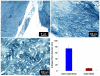
(a) Transmission electron microscopy images of collagen ultrastructure in woven scaffold seeded with cells at day 35. The core region of ELAC thread is highlighted as ‘B’ and the surface of the thread is highlighted with ‘C’ close to which an elongated cell is present. (b) Morphology of collagen fibrils of ELAC thread, and c) collagen fibers on thread surfaces synthesized by cells at day 35 displayed d-banding pattern which was absent in the fibrils of ELAC threads (b). (d) Average diameters of collagen fibers in ELAC thread and cell produced collagen. The horizontal line indicates significant difference (p<0.05).
Similar articles
- Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads.
Kishore V, Bullock W, Sun X, Van Dyke WS, Akkus O. Kishore V, et al. Biomaterials. 2012 Mar;33(7):2137-44. doi: 10.1016/j.biomaterials.2011.11.066. Epub 2011 Dec 15. Biomaterials. 2012. PMID: 22177622 Free PMC article. - Mesenchymal Stem Cell Delivery via Topographically Tenoinductive Collagen Biotextile Enhances Regeneration of Segmental Tendon Defects.
McClellan P, Ina JG, Knapik DM, Isali I, Learn G, Valente A, Wen Y, Wen R, Anderson JM, Gillespie RJ, Akkus O. McClellan P, et al. Am J Sports Med. 2022 Jul;50(8):2281-2291. doi: 10.1177/03635465221097939. Epub 2022 Jun 1. Am J Sports Med. 2022. PMID: 35647785 Free PMC article. - Woven collagen biotextiles enable mechanically functional rotator cuff tendon regeneration during repair of segmental tendon defects in vivo.
Learn GD, McClellan PE, Knapik DM, Cumsky JL, Webster-Wood V, Anderson JM, Gillespie RJ, Akkus O. Learn GD, et al. J Biomed Mater Res B Appl Biomater. 2019 Aug;107(6):1864-1876. doi: 10.1002/jbm.b.34279. Epub 2018 Nov 28. J Biomed Mater Res B Appl Biomater. 2019. PMID: 30485649 Free PMC article. - Tenogenic differentiation of stem cells for tendon repair-what is the current evidence?
Lui PP, Rui YF, Ni M, Chan KM. Lui PP, et al. J Tissue Eng Regen Med. 2011 Aug;5(8):e144-63. doi: 10.1002/term.424. Epub 2011 May 5. J Tissue Eng Regen Med. 2011. PMID: 21548133 Review. - In the beginning there were soft collagen-cell gels: towards better 3D connective tissue models?
Brown RA. Brown RA. Exp Cell Res. 2013 Oct 1;319(16):2460-9. doi: 10.1016/j.yexcr.2013.07.001. Epub 2013 Jul 12. Exp Cell Res. 2013. PMID: 23856376 Review.
Cited by
- Electrobiofabrication: electrically based fabrication with biologically derived materials.
Li J, Wu S, Kim E, Yan K, Liu H, Liu C, Dong H, Qu X, Shi X, Shen J, Bentley WE, Payne GF. Li J, et al. Biofabrication. 2019 Apr 26;11(3):032002. doi: 10.1088/1758-5090/ab06ea. Biofabrication. 2019. PMID: 30759423 Free PMC article. Review. - Braided and Stacked Electrospun Nanofibrous Scaffolds for Tendon and Ligament Tissue Engineering.
Rothrauff BB, Lauro BB, Yang G, Debski RE, Musahl V, Tuan RS. Rothrauff BB, et al. Tissue Eng Part A. 2017 May;23(9-10):378-389. doi: 10.1089/ten.TEA.2016.0319. Epub 2017 Feb 10. Tissue Eng Part A. 2017. PMID: 28071988 Free PMC article. - Augmentation of Tendon and Ligament Repair with Fiber-Reinforced Hydrogel Composites.
Kent RN 3rd, Huang AH, Baker BM. Kent RN 3rd, et al. Adv Healthc Mater. 2024 Nov;13(29):e2400668. doi: 10.1002/adhm.202400668. Epub 2024 Aug 12. Adv Healthc Mater. 2024. PMID: 39135411 Free PMC article. Review. - Collagen Substrate Stiffness Anisotropy Affects Cellular Elongation, Nuclear Shape, and Stem Cell Fate toward Anisotropic Tissue Lineage.
Islam A, Younesi M, Mbimba T, Akkus O. Islam A, et al. Adv Healthc Mater. 2016 Sep;5(17):2237-47. doi: 10.1002/adhm.201600284. Epub 2016 Jul 5. Adv Healthc Mater. 2016. PMID: 27377355 Free PMC article. - Insight and Recent Advances into the Role of Topography on the Cell Differentiation and Proliferation on Biopolymeric Surfaces.
Tudureanu R, Handrea-Dragan IM, Boca S, Botiz I. Tudureanu R, et al. Int J Mol Sci. 2022 Jul 13;23(14):7731. doi: 10.3390/ijms23147731. Int J Mol Sci. 2022. PMID: 35887079 Free PMC article. Review.
References
- Tan SL, Ahmad RE, Ahmad TS, Merican AM, Abbas AA, Ng WM, Kamarul T. Cells, Tissues, Organs. 2012;196:325. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous