Buffer mobility and the regulation of neuronal calcium domains - PubMed (original) (raw)
Review
Buffer mobility and the regulation of neuronal calcium domains
Elizabeth A Matthews et al. Front Cell Neurosci. 2015.
Abstract
The diffusion of calcium inside neurons is determined in part by the intracellular calcium binding species that rapidly bind to free calcium ions upon entry. It has long been known that some portion of a neuron's intracellular calcium binding capacity must be fixed or poorly mobile, as calcium diffusion is strongly slowed in the intracellular environment relative to diffusion in cytosolic extract. The working assumption was that these immobile calcium binding sites are provided by structural proteins bound to the cytoskeleton or intracellular membranes and may thereby be relatively similar in composition and capacity across different cell types. However, recent evidence suggests that the immobile buffering capacity can vary greatly between cell types and that some mobile calcium binding proteins may alter their mobility upon binding calcium, thus blurring the line between mobile and immobile. The ways in which immobile buffering capacity might be relevant to different calcium domains within neurons has been explored primarily through modeling. In certain regimes, the presence of immobile buffers and the interaction between mobile and immobile buffers have been shown to result in complex spatiotemporal patterns of free calcium. In total, these experimental and modeling findings call for a more nuanced consideration of the local intracellular calcium microenvironment. In this review we focus on the different amounts, affinities, and mobilities of immobile calcium binding species; propose a new conceptual category of physically diffusible but functionally immobile buffers; and discuss how these buffers might interact with mobile calcium binding partners to generate characteristic calcium domains.
Keywords: calcium buffer; calcium domains; diffusion coefficient; immobile; mobile.
Figures
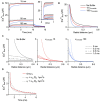
FIGURE 1
Simulations were run using CalC (Matveev et al., 2004; http://web.njit.edu/\~matveev; Version 6.7.4). Resting calcium concentration was set at 100 nM. We simulate a calcium current of 0.2 pA through a generic calcium channel. Parameters for the immobile buffer were taken from the experimental results summarized in Table 1. (A) The presence of immobile buffer slows the arrival at steady state free calcium concentrations at all points in space surrounding a calcium source. The time of channel opening was increased to a non-physiological 10 ms so that the prolonged time course could be better illustrated. However, in the case of high immobile buffering capacity, steady state concentrations were clearly not reached even after 10 ms. In the case of a more physiologically relevant open time of 0.8 ms, the free calcium concentration is even further from reaching a steady state level and the slowing effect of immobile buffers is even more prominent (inset). A similar effect is seen after the channel closes. Calcium is gradually released from immobile buffers, prolonging the return to resting calcium concentrations. (B) The localizing effect of immobile buffers on microdomains during calcium entry was plotted for a 1 ms calcium channel opening. At all illustrated points in space, the immobile buffer reduces free calcium concentration; this localizing effect on peak free calcium concentration is known to be similar for a mobile buffer. (C) The collapse of a microdomain after the closing of a calcium channel is illustrated for conditions with no buffering (left), a low immobile buffer capacity (center) and a high immobile buffer capacity (right). The spatial calcium gradient is shown immediately before closing (0 ms), and at 0.2, 0.5, and 1.0 ms following channel closure. The strong prolongation of the free calcium domain by the immobile buffer is apparent. With a high immobile buffer capacity, the domain remains above 1 μM free calcium even 1 ms after the channel has closed. This effect is unique to immobile buffers; a mobile buffer will disperse the spatial gradient and speed the local return to resting levels. (D) To explore the diffusion limits at which a mobile buffer behaves as a functionally immobile buffer, the prolongation of a calcium microdomain was examined. A calcium source was opened for 1 ms, then the decay of the microdomain at 15 nm from the channel was plotted over time. The initial condition had an immobile buffer with a capacity of 15; under these conditions, the free calcium concentration remains elevated for several milliseconds. When the diffusion coefficient was ≤ 1 μm2/s, the lifetime was further increased by the mobile buffer. This indicates that the poorly mobile buffer functions as though it were physically immobile.
Similar articles
- Cytoplasmic Calcium Buffering: An Integrative Crosstalk.
Gilabert JA. Gilabert JA. Adv Exp Med Biol. 2020;1131:163-182. doi: 10.1007/978-3-030-12457-1_7. Adv Exp Med Biol. 2020. PMID: 31646510 Review. - Mobile and immobile calcium buffers in bovine adrenal chromaffin cells.
Zhou Z, Neher E. Zhou Z, et al. J Physiol. 1993 Sep;469:245-73. doi: 10.1113/jphysiol.1993.sp019813. J Physiol. 1993. PMID: 8271200 Free PMC article. - Tuning local calcium availability: cell-type-specific immobile calcium buffer capacity in hippocampal neurons.
Matthews EA, Schoch S, Dietrich D. Matthews EA, et al. J Neurosci. 2013 Sep 4;33(36):14431-45. doi: 10.1523/JNEUROSCI.4118-12.2013. J Neurosci. 2013. PMID: 24005295 Free PMC article. - Physiology of intracellular calcium buffering.
Eisner D, Neher E, Taschenberger H, Smith G. Eisner D, et al. Physiol Rev. 2023 Oct 1;103(4):2767-2845. doi: 10.1152/physrev.00042.2022. Epub 2023 Jun 16. Physiol Rev. 2023. PMID: 37326298 Free PMC article. Review.
Cited by
- Rethinking calcium profiles around single channels: the exponential and periodic calcium nanodomains.
Mironov SL. Mironov SL. Sci Rep. 2019 Nov 20;9(1):17196. doi: 10.1038/s41598-019-53095-4. Sci Rep. 2019. PMID: 31748584 Free PMC article. - The spatio-temporal properties of calcium transients in hippocampal pyramidal neurons in vitro.
Shkryl VM. Shkryl VM. Front Cell Neurosci. 2022 Dec 14;16:1054950. doi: 10.3389/fncel.2022.1054950. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36589284 Free PMC article. - Making sense of astrocytic calcium signals - from acquisition to interpretation.
Semyanov A, Henneberger C, Agarwal A. Semyanov A, et al. Nat Rev Neurosci. 2020 Oct;21(10):551-564. doi: 10.1038/s41583-020-0361-8. Epub 2020 Sep 1. Nat Rev Neurosci. 2020. PMID: 32873937 Review. - Nano-organization of synaptic calcium signaling.
McCarthy CI, Kavalali ET. McCarthy CI, et al. Biochem Soc Trans. 2024 Jun 26;52(3):1459-1471. doi: 10.1042/BST20231385. Biochem Soc Trans. 2024. PMID: 38752834 Free PMC article. Review. - A polybasic motif in alternatively spliced KChIP2 isoforms prevents Ca2+ regulation of Kv4 channels.
Murphy JG, Hoffman DA. Murphy JG, et al. J Biol Chem. 2019 Mar 8;294(10):3683-3695. doi: 10.1074/jbc.RA118.006549. Epub 2019 Jan 8. J Biol Chem. 2019. PMID: 30622142 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous