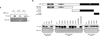Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway - PubMed (original) (raw)
Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway
Jung-Soon Mo et al. Nat Cell Biol. 2015 Apr.
Abstract
YAP (Yes-associated protein) is a transcription co-activator in the Hippo tumour suppressor pathway and controls cell growth, tissue homeostasis and organ size. YAP is inhibited by the kinase Lats, which phosphorylates YAP to induce its cytoplasmic localization and proteasomal degradation. YAP induces gene expression by binding to the TEAD family transcription factors. Dysregulation of the Hippo-YAP pathway is frequently observed in human cancers. Here we show that cellular energy stress induces YAP phosphorylation, in part due to AMPK-dependent Lats activation, thereby inhibiting YAP activity. Moreover, AMPK directly phosphorylates YAP Ser 94, a residue essential for the interaction with TEAD, thus disrupting the YAP-TEAD interaction. AMPK-induced YAP inhibition can suppress oncogenic transformation of Lats-null cells with high YAP activity. Our study establishes a molecular mechanism and functional significance of AMPK in linking cellular energy status to the Hippo-YAP pathway.
Figures
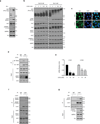
Figure 1. Cellular energy starvation activates Lats and inhibits YAP
(a) Glucose starvation induces YAP phosphorylation. HEK293A cells were starved of glucose as indicated for 1 hr. Cell lysates were prepared and subjected to immunoblotting for the indicated proteins and phosphorylation. Phos-tag denotes phos-tag gel used to resolve phosphorylated YAP based on mobility shift. Vin stands for vinculin as a loading control. Protein molecular markers are labeled on the right of each panel. (b) 2-DG induces YAP phosphorylation. HEK293A cells were treated with different doses of 2-DG in glucose-rich (25 mM) or glucose-free (0 mM) DMEM medium for 1 hr. Cell lysates were immunoblotted with anti-AMP-activated protein kinase α (AMPKα), anti-pAMPKα (T172), anti-Acetyl-CoA Carboxylase (ACC), and anti-pACC (S79). Both pAMPKα and pACC were analyzed as indicators of energy starvation. Experiments were similar to panel a. (c) 2-DG decreases YAP nuclear localization. HEK293A cells were treated with 25 mM 2-DG for 1 hr. YAP subcellular localization was determined by immunofluorescence staining for endogenous YAP (green); DAPI (blue) was used to stain cell nuclei. Scale Bars, 10µm. (d) 2-DG disrupts the interaction between YAP and TEAD1. Cells were treated with 25 mM 2-DG for 2 hr as indicated. Cell lysates were precipitated with YAP or IgG control antibody. Co-immunoprecipitation of TEAD was detected by Western blot. (e) 2-DG inhibits YAP target gene expression. HEK293A cells were treated with 10 mM and 25 mM 2-DG for 4 hr, mRNA levels of CTGF and CYR61 were measured by quantitative RT-PCR and normalized to HPRT control (error bars represent ± s.e.m. from n=3 independent experiments). (f) Energy stress increased Lats1 phosphorylation. HEK293A cells were treated with 25 mM 2-DG for 1 hr. Endogenous Lats1 was immunoprecipitated and phosphorylation of the activation loop and hydrophobic motif was detected by Western blot with phosphospecific antibodies. (g) Glucose starvation activates Lats1 kinase activity. Cells were treated with glucose starvation for 1 hr. Lats1 was immunoprecipitated and in vitro kinase assays were performed using recombinant GST-YAP as the substrate. Phosphorylation of GST-YAP was determined by immunoblotting with the phospho-YAP (S127) antibody. All blots and immunofluorescence shown; are representatives of three independent experiments. Uncropped blots are shown in Supplementary Fig. 5. ***P<0.001, two-tailed _t_-test.
Figure 2. AMPK is required for Hippo-YAP regulation by energy stress
(a) The 2-DG-induced YAP phosphorylation depends on AMPK. AMPKα WT and AMPKα DKO MEFs were treated with 25 mM 2-DG for the indicated durations. YAP phosphorylation is determined by phos-tag gel. (b) AMPK induces YAP phosphorylation. HEK293A cells were transiently co-transfected with the indicated plasmids as labeled on top of the lanes. Wild type AMPKα (AMPKα WT), but not the kinase-inactive AMPKα (AMPKα DN) increased YAP phosphorylation. (c) AMPK is required for Lats1 activation by energy stress. AMPKα WT and AMPKα DKO MEFs were incubated with or without 25 mM 2-DG for 2 hr. Endogenous Lats1 was immunoprecipitated and phosphorylation in both activation loop and the hydrophobic motif was detected by Western blot. (d) AMPK disrupts YAP and TEAD1 interaction. HEK293A cells were transiently co-transfected with the indicated plasmids. Flag-YAP was immunoprecipitated and the co-immunoprecipitated Myc-TEAD1 was detected by Myc Western blot. (e) AMPK inhibits the TEAD reporter. A luciferase reporter controlled by multiple TEAD binding sequences was transfected in to HEK293A cells together with AMPKα WT or AMPKα DN. After 48 hr, the firefly luciferase activity was measured and normalized to the co-transfected Renilla luciferase internal control (error bars represent ± s.e.m. from n=6 biological replicates). (f) Metformin activates AMPK and increases YAP phosphorylation. Primary mouse hepatocytes were treated with different doses of metformin for 4 or 8 hr. Phosphorylation of YAP and AMPK were measured by Western blot. TAZ phosphorylation was indicated by a mobility shift. (g) AICAR disrupts YAP and TEAD interaction. Primary mouse hepatocytes were treated with 2 mM AICAR for 4 hr. The endogenous YAP was immunoprecipitated and the co-precipitated TEAD was detected by Western blot. (h) AICAR and metformin inhibits YAP target gene expression. Primary mouse hepatocytes were treated with 2 mM AICAR or 2 mM metformin for 4 hr, mRNA levels of Ctgf and Cyr61 were measured by quantitative RT-PCR (error bars represent ± s.e.m. from n=3 independent experiments). All experiments are representatives of three independent experiments except panel e. *P<0.05, **P<0.01, two-tailed _t_-test.
Figure 3. Energy stress induces YAP phosphorylation via both Lats dependent and independent mechanisms
(a) Deletion of Lats1/2 diminishes, but not completely abolishes YAP phosphorylation by 2-DG. Lats1−/−;Lats2fl/fl (denoted as Lats1 KO) MEFs and Cre- transduced Lats1−/−;Lats2fl/fl (denoted as Lst1/2 DKO) MEFs were treated with 10 and 25 mM 2-DG. YAP phosphorylation was detected by phospho-YAP (S127) antibody and phos-tag gel. Lats2 deletion was confirmed by Western blot. All lysates were run on the same gel and the vertical line in YAP phos-tag blot indicated different exposures of the same Western Blot (the YAP phos-tag blot of Lats1/2 DKO samples was exposed shorter due to high YAP protein levels in these samples). (b) Overexpressed of kinase-inactive Lats2 (Lats2-KR) abolishes 2-DG-induced S127 phosphorylation, but not total phosphorylation, in YAP. Flag-YAP was co-transfected with Lats2-WT or Lats2-KR mutant. After transfection, cells were treated with 25 mM 2-DG for 1 hr. (c) AMPK induces mobility shift of YAP-5SA. Flag-YAP-5SA was co-transfected with AMPKα WT or AMPKα DN in HEK293A as indicated. (d, e) 2-DG stimulates the interaction of YAP and AMPKα. HEK293A cells transfected with the indicated plasmids were immunoprecipitated with anti-FLAG (for Flag-YAP) or anti-HA (for HA-AMPK) and blotted with indicated antibodies for co-immunoprecipitation. (f) AICAR or Metformin promotes the association between endogenous AMPK and YAP. H2.35 cells were treated with AICAR or metformin. Cell lysates were immunoprecipitated with YAP or control IgG and Western blotted with AMPKα. All experiments are representatives of three independent experiments except panel e and f. The experiments in e, f were performed two times with similar results.
Figure 4. AMPK phosphorylates S94 in YAP
(a) AMPK directly phosphorylates YAP in vitro. The indicated various YAP mutant proteins were purified from E.coli and used as substrates for in vitro phosphorylation by AMPK. Phosphorylation was determined by 32P-autoradiography and the amount of each GST-YAP protein was detected by coomassie staining. (b) Confirmation of AMPK phosphorylation sites in YAP by mutagenesis. Schematic representation of YAP domains and deletion constructs used to map phosphorylation sites are shown on the top. The human YAP protein contains a TEAD Binding Domain (TBD; residues 48–102), two WW domains (WW1, 171–204; WW2, 230–263) domains and an Activation Domain (AD; 292–488). Three different YAP deletion constructs and multiple amino acid mutations in these YAP truncations were expressed, purified, and used as substrates for in vitro AMPK phosphorylation in the presence of 32P-ATP. Phosphorylation was detected by autoradiography. GST-YAP fragment protein was detected by Coomassie staining.
Figure 5. AMPK inhibits YAP activity through phosphorylation of serine 94
(a) Phosphomimetic mutant of YAP S94 abolishes YAP activity. The indicated plasmids were co-transfected with a 5×UAS-luciferase reporter for Gal4-TEAD4 and Renilla construct into HEK293T cells. The firefly luciferase activity levels were measured and normalized to Renilla luciferase activity levels (error bars represent ± s.e.m. from n=6 biological replicates). (b) S94 is important for YAP activity and inhibition by AMPK. All three AMPK phosphorylation sites in YAP were mutated to alanine. The YAP plasmids were co-transfected with the 5xUAS-luciferase reporter for Gal4-TEAD4 into HEK293T cells together with the control Renilla luciferase. After 48 hr, the firefly luciferase activity was measured and normalized to the co-transfected Renilla luciferase internal control (error bars represent ± s.e.m. from n=6 biological replicates). (c) S94 of YAP is essential for 2-DG-induced disruption of YAP-TEAD complex. HEK293A cells were transiently co-transfected with the indicated plasmids followed by treatment with 2-DG. Interaction between Flag-YAP and Myc-TEAD4 was determined by co-immunoprecipitation. (d) Evaluation of phospho-specific antibodies for YAP S94 phosphorylation. Phosphoantibody was prepared by immunizing rabbits with synthetic phosphopeptides containing phospho-YAP S94. Recombinant GST-YAP (51–270) fragment was purified from bacteria and phosphorylated by AMPK in vitro. After the reaction, 5 ng of the GST-YAP protein was detected by phospho-YAP S94 or GST antibody. The non-phosphorylatable mutant, GST-YAP-S94A was used as a negative control. (e) AMPK increases YAP S94 phosphorylation in transfected cells. Flag-YAP WT and YAP-S94A mutant were transfected into HEK293 cells with or without AMPK as indicated. Flag-YAP was immunoprecipitated and phosphorylation of S94 was detected by the pYAP(S94) phosphoantibody. (f) AICAR increases YAP S94 phosphorylation in vivo. Primary hepatocytes were treated with 2 mM AICAR for 4 hr. YAP was immunoprecipitated and phosphorylation of S94 was detected by the pYAP(S94) phosphoantibody. All blots shown are representatives from three independent experiments except panel e. **P<0.01, ***P<0.001, two-tailed _t_-test.
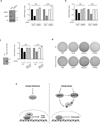
Figure 6. Energy stress attenuates tumorigenecity of Lats1/2 DKO MEFs
(a) AICAR inhibits YAP target gene expression in Lats1/2 DKO MEFs. Lats1/2 DKO MEFs were treated with 0.5 mM and 1 mM of AICAR for 5 hr. mRNA levels of Ctgf and Cyr61 were measured by quantitative RT-PCR (error bars represent ± s.e.m. from n=3 independent experiments). (b) Knockdown of YAP or TAZ attenuates anchorage-independent growth of Lats1/2 DKO MEFs. YAP or TAZ was knockdown by shRNAs in Lats1/2 DKO MEFs (the experiments was repeated 2 times). The Lats1/2 DKO MEFs (3 × 103 cells) were seeded onto a culture medium containing 0.3% agar and incubated at 37 °C for 2 weeks. The colonies were stained with 0.005% crystal violet. (c) Energy stress attenuates anchorage-independent growth of Lats1/2 DKO MEFs. Lats1/2 DKO MEFs (3 × 103 cells) were seeded onto a culture medium containing 0.3% agar and incubated at 37 °C for 2 weeks. The colonies were stained with 0.005% crystal violet and quantified. AICAR and metformin treatments were indicated (error bars represent ± s.e.m. from n=3 independent experiments). (d) Metformin inhibits tumor growth of Lats1/2 DKO MEFs in xenografted mice. Mice were ectopically implanted with 1x106 cells on the both sides of each mouse. Daily i.p. injection of 250mg/kg dose of Metformin or equal volume (300µL) of vehicle (PBS) for 15 days. At the end of the experiment, mice were sacrificed 16 days after implantation and tumor weight was measured (error bars represent ± s.e.m. from 8 tumors (4 mice) per group). Scale bar, 1cm. *P<0.05, two-tailed _t_-test.
Figure 7. AMPK is required for energy stress to inhibit YAP activity
(a) AMPK is required for inhibition of Ctgf and Cry61 by AICAR. Lats1/2 DKO MEFs were transfected with control or AMPK siRNAs. The knockdown efficiency of AMPK was confirmed by Western blot (left panels). After treating with the AMPK activator AICAR (0.5 mM), mRNA levels of Ctgf and Cyr61 were measured by quantitative RT-PCR. The mRNA relative unit values are normalized to 1 (controls without AICAR treatment) (the experiments was repeated 2 times). (b) Lats1/2 DKO MEFs were transiently transfected with control (siCon) or AMPK (siAMPKa) siRNA. After treating with the AMPK activator A769662 (0.3mM for 4 hr), mRNA levels of Ctgf and Cyr61 were measured by quantitative RT-PCR (the experiments was repeated 2 times). (c) AICAR cannot inhibit YAP target genes in cells expressing TEAD1ΔC-YAP(AD). HA-TEAD1ΔC-YAP(AD), which is a fusion of the TEAD DNA binding domain with the YAP transactivation domain, was stably expressed in Lats1/2 DKO MEFs. Expression of HA- TEAD1ΔC-YAP(AD) was confirmed by Western blot. Cells were treated in AICAR as indicated and mRNA levels of Ctgf and Cyr61 were measured by quantitative RT-PCR (error bars represent ± s.e.m. from n=5 independent experiments). (d) Expression of HA-TEAD1ΔC-YAP(AD) confers Lats1/2 DKO MEFs resistant to growth inhibition by AMPK activators. Soft-agar colony-formation assay was performed with Lats1/2 DKO MEFs expressed with vector (top row) or HA- TEAD1ΔC-YAP(AD) (bottom row). Treatments with PBS control, AICAR, metformin, or A769662 are indicated. (e) A model for regulation of Hippo-YAP pathway by energy stress and AMPK. When the energy is sufficient, AMPK is inactive and YAP is active. When cellular energy level is low (energy stress), Both AMPK and Lats are active. The active AMPK and Lats inhibit YAP by phosphorylation.
Similar articles
- Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation.
Lin KC, Moroishi T, Meng Z, Jeong HS, Plouffe SW, Sekido Y, Han J, Park HW, Guan KL. Lin KC, et al. Nat Cell Biol. 2017 Jul 28;19(8):996-1002. doi: 10.1038/ncb3581. Nat Cell Biol. 2017. PMID: 28752853 Free PMC article. - Tumor suppressor LATS1 is a negative regulator of oncogene YAP.
Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Hao Y, et al. J Biol Chem. 2008 Feb 29;283(9):5496-509. doi: 10.1074/jbc.M709037200. Epub 2007 Dec 24. J Biol Chem. 2008. PMID: 18158288 - The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity.
Shimomura T, Miyamura N, Hata S, Miura R, Hirayama J, Nishina H. Shimomura T, et al. Biochem Biophys Res Commun. 2014 Jan 17;443(3):917-23. doi: 10.1016/j.bbrc.2013.12.100. Epub 2013 Dec 28. Biochem Biophys Res Commun. 2014. PMID: 24380865 - Control of YAP/TAZ Activity by Metabolic and Nutrient-Sensing Pathways.
Santinon G, Pocaterra A, Dupont S. Santinon G, et al. Trends Cell Biol. 2016 Apr;26(4):289-299. doi: 10.1016/j.tcb.2015.11.004. Epub 2015 Dec 30. Trends Cell Biol. 2016. PMID: 26750334 Review. - The Hippo Pathway and YAP/TAZ-TEAD Protein-Protein Interaction as Targets for Regenerative Medicine and Cancer Treatment.
Santucci M, Vignudelli T, Ferrari S, Mor M, Scalvini L, Bolognesi ML, Uliassi E, Costi MP. Santucci M, et al. J Med Chem. 2015 Jun 25;58(12):4857-73. doi: 10.1021/jm501615v. Epub 2015 Mar 11. J Med Chem. 2015. PMID: 25719868 Review.
Cited by
- Catechol enhances chemo‑ and radio‑sensitivity by targeting AMPK/Hippo signaling in pancreatic cancer cells.
Moon JY, Ediriweera MK, Ryu JY, Kim HY, Cho SK. Moon JY, et al. Oncol Rep. 2021 Mar;45(3):1133-1141. doi: 10.3892/or.2021.7924. Epub 2021 Jan 5. Oncol Rep. 2021. PMID: 33650657 Free PMC article. - Activation of AMPK inhibits Galectin-3-induced pulmonary artery smooth muscle cells proliferation by upregulating hippo signaling effector YAP.
Zhang Q, Li W, Zhu Y, Wang Q, Zhai C, Shi W, Feng W, Wang J, Yan X, Chai L, Chen Y, Li C, Liu P, Li M. Zhang Q, et al. Mol Cell Biochem. 2021 Aug;476(8):3037-3049. doi: 10.1007/s11010-021-04131-3. Epub 2021 Apr 2. Mol Cell Biochem. 2021. PMID: 33797701 - AMPKα1 deletion in fibroblasts promotes tumorigenesis in athymic nude mice by p52-mediated elevation of erythropoietin and CDK2.
Zhou Y, Xu H, Ding Y, Lu Q, Zou MH, Song P. Zhou Y, et al. Oncotarget. 2016 Aug 16;7(33):53654-53667. doi: 10.18632/oncotarget.10687. Oncotarget. 2016. PMID: 27449088 Free PMC article. - TNS1: Emerging Insights into Its Domain Function, Biological Roles, and Tumors.
Wang Z, Ye J, Dong F, Cao L, Wang M, Sun G. Wang Z, et al. Biology (Basel). 2022 Oct 26;11(11):1571. doi: 10.3390/biology11111571. Biology (Basel). 2022. PMID: 36358270 Free PMC article. Review. - A Potential Role of YAP/TAZ in the Interplay Between Metastasis and Metabolic Alterations.
Yamaguchi H, Taouk GM. Yamaguchi H, et al. Front Oncol. 2020 Jun 11;10:928. doi: 10.3389/fonc.2020.00928. eCollection 2020. Front Oncol. 2020. PMID: 32596154 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- DE015964/DE/NIDCR NIH HHS/United States
- EY022611/EY/NEI NIH HHS/United States
- R01 EY022611/EY/NEI NIH HHS/United States
- R01 CA132809/CA/NCI NIH HHS/United States
- CA132809/CA/NCI NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- CA23100/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases