A mouse model of L-2-hydroxyglutaric aciduria, a disorder of metabolite repair - PubMed (original) (raw)
A mouse model of L-2-hydroxyglutaric aciduria, a disorder of metabolite repair
Rim Rzem et al. PLoS One. 2015.
Abstract
The purpose of the present work was to progress in our understanding of the pathophysiology of L-2-hydroxyglutaric aciduria, due to a defect in L-2-hydroxyglutarate dehydrogenase, by creating and studying a mouse model of this disease. L-2-hydroxyglutarate dehydrogenase-deficient mice (l2hgdh-/-) accumulated L-2-hydroxyglutarate in tissues, most particularly in brain and testis, where the concentration reached ≈ 3.5 μmol/g. Male mice showed a 30% higher excretion of L-2-hydroxyglutarate compared to female mice, supporting that this dicarboxylic acid is partially made in males by lactate dehydrogenase C, a poorly specific form of this enzyme exclusively expressed in testes. Involvement of mitochondrial malate dehydrogenase in the formation of L-2-hydroxyglutarate was supported by the commensurate decrease in the formation of this dicarboxylic acid when down-regulating this enzyme in mouse l2hgdh-/- embryonic fibroblasts. The concentration of lysine and arginine was markedly increased in the brain of l2hgdh-/- adult mice. Saccharopine was depleted and glutamine was decreased by ≈ 40%. Lysine-α-ketoglutarate reductase, which converts lysine to saccharopine, was inhibited by L-2-hydroxyglutarate with a Ki of ≈ 0.8 mM. As low but significant activities of the bifunctional enzyme lysine-α-ketoglutarate reductase/saccharopine dehydrogenase were found in brain, these findings suggest that the classical lysine degradation pathway also operates in brain and is inhibited by the high concentrations of L-2-hydroxyglutarate found in l2hgdh-/- mice. Pathological analysis of the brain showed significant spongiosis. The vacuolar lesions mostly affected oligodendrocytes and myelin sheats, as in other dicarboxylic acidurias, suggesting that the pathophysiology of this model of leukodystrophy may involve irreversible pumping of a dicarboxylate in oligodendrocytes. Neurobehavioral testing indicated that the mice mostly suffered from a deficit in learning capacity. In conclusion, the findings support the concept that L-2-hydroxyglutaric aciduria is a disorder of metabolite repair. The accumulation of L-2-hydroxyglutarate exerts toxic effects through various means including enzyme inhibition and glial cell swelling.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
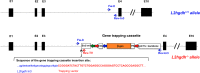
Fig 1. Generation of the murine l2hgdh -/- model by insertion of a gene trapping cassette.
We show the genomic structure of the l2hgdh gene in wild-type and knock-out mice, which spans 10 exons (black boxes). In the disrupted allele, the gene trapping cassette [containing engrailed intron1 (En2 int1); Lox71 and LoxP Cre recombination sites (green and blue arrows); FRT flippase recombination sites (red arrows); β-geo cassette (orange box); splicing acceptor (SA) and polyadenylation (PA) sequences; a portion of pGTX1 vector (pUC backbone)] has been randomly inserted in intron 3, which leads to the production of an inactive protein comprising the first 137 residues of L-2-hydroxyglutarate dehydrogenase fused to the β-geo cassette. The latter confers resistance to neomycin and interrupts downstream transcription due to the addition of a polyadenylation signal. The precise site of insertion of the gene-trapping cassette is shown, as well as the location of the primers (Fw-8; Rev-In3; Rev-TV) used for diagnostic amplification of the wild-type and mutated alleles.
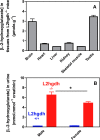
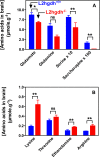
Fig 2. L-2-hydroxyglutarate concentration in different tissues (A) and its urinary excretion (B) in l2hgdh +/+ and l2hgdh -/- mice.
Tissues of l2hgdh -/- male mice (n = 4) were analyzed in (A). Values of urinary excretion for male and female mice (n = 3) are shown in (B). Results are means ± SEM. * p < 0.05 by unpaired t test.
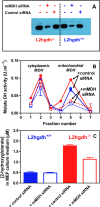
Fig 3. L-2-hydroxyglutarate formation in l2hgdh -/- cells is decreased if mitochondrial malate dehydrogenase (mMDH) is knocked down.
MEF cells derived from l2hgdh +/+ and l2hgdh -/- 14.5 day-embryos were treated with control (scrambled) or mMDH-specific siRNAs for 48 h. (A) mMDH immunoreactivity was determined in lysates of cells treated or not with siRNA. (B) mMDH activity in cell lysates was determined by separating this enzyme from cytoplasmic MDH by cation exchange chromatography. (C) (D+L)-2-hydroxyglutarate concentration was determined in the medium by GC-MS.
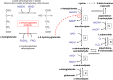
Fig 4. Formation and breakdown of L-2-hydroxyglutarate and interference of this compound with lysine metabolism.
The scheme shows how L-2-hydroxyglutarate is formed and degraded. It also shows the initial steps of the major lysine catabolic pathway (via saccharopine) present in mammalian tissues and of the minor pathway (via L-pipecolate) present in brain and the inhibition exerted by L-2-hydroxyglutarate. 1. Lysine-α-ketoglutarate reductase; 2. Saccharopine dehydrogenase; 3. α-Aminoadipate semialdehyde dehydrogenase; 4. α-Aminoadipate transaminase; 5. Imine reductase; 6. L-Pipecolate oxidase.
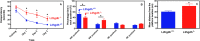
Fig 5. Decreased (A) or increased (B) concentrations of brain amino acids of l2hgdh -/- mice compared to l2hgdh +/+ mice.
Brains from 24 h fasted mice aged 4–6 months were analyzed. ‘Serine x 10’ and ‘Saccharopine x 100’ indicate that the actual concentrations in brain is 10-fold or 100-fold lower, respectively, compared to the values that are shown in the Y axis. Results are the means ± SEM for n = 10 (l2hgdh +/+) or n = 6 (l2hgdh -/-) mice. *: 0.0003 < alpha < 0.0016; **: alpha < 0.0003; ns: non significant. The analysis was performed using the Student t-test with a Bonferroni correction for multiple testing (see also Table 1).
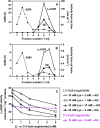
Fig 6. Presence of the bifunctional enzyme lysine-α-ketoglutarate reductase/saccharopine dehydrogenase in brain (A, B) and inhibition of lysine-α-ketoglutarate reductase by L-2-hydroxyglutarate (C).
(A) and (B) show the elution profile of lysine-α-ketoglutarate reductase (LαKGR) and saccharopine dehydrogenase (SD) from Blue Trisacryl columns on which a mouse liver (A) or a mouse brain (B) extract have been applied. (C) Partially purified lysine-α-ketoglutarate reductase from mouse liver was assayed at 30°C in the presence of 25 mM Hepes, pH 8.0, 0.15 mM NADPH, 1 mM dithiothreitol, 10 mM lysine (Lys) and 2, 5 or 10 mM α-ketoglutarate (α-KG) as indicated and increasing concentrations of L-2-hydroxyglutarate. The effect of L-2-hydroxyglutarate was also tested in the presence of 25 mM lysine and 2 mM α-ketoglutarate and that of D-2-hydroxyglutarate with 10 mM lysine and 2 mM α-ketoglutarate.
Fig 7. Pathological analysis of l2hgdh -/- mice.
The figure compares the histological and ultrastructural appearance of the brain of l2hgdh +/+ (left panels: A, B, E, G, H, K, L) and l2hgdh -/- mice (right panels: C, D, F, I, J, M, N). Hematoxylin and eosin staining shows low-magnification of the lateral part of the brain (A, C) at the level of the lateral ventricle (LV), the caudate putamen (striatum; CPu) and the anterior commissure, anterior part (aca). Higher magnification of pencils of Wilson are shown in panels B and D. Notice the satellite oligodendrocyte at B (arrow). Luxol fast blue staining of myelin is shown in E and F, highlighting the predominant presence of vacuoles in the pencils of Wilson of the striatum of the l2hgdh -/- mouse. Panels G-J are microphotographs from the junction between cortex and white matter in the vicinity of corpus callosum. Immunostaining of NeuN (G and I) shows that most vacuoles in the l2hgdh -/- mice are at distance from the nucleus of neurons (notice the abundance of vacuoles in the lower part of panel I, devoid of neurons) although a few vacuoles indent the nucleus of some neurons (arrowheads). However, the nucleus of satellite oligodendrocytes is also present in close contact to the vacuole indenting the nucleus of some neurons (arrows), preventing to identify clearly which cell type actually contains the peri-neuronal vacuole. In contrast, immunostaining of Olig2 (H and J) clearly shows that most vacuoles are in close contact to the nucleus of oligodendrocytes in the l2hgdh -/- mouse brain. Notice the small size of the nucleus of oligodendrocytes compared to the nucleus of neurons. Panels K-N are Transmission Electron Microscopy photographs of striatum tissue. Ultrastructural analysis (K-N) shows many empty-looking cell processes (asterisks in panels M and N), containing dilated cytoplasmic organelles, in the glia of l2hgdh -/- mice. The myelin sheath of many axons appears focally altered or disrupted (arrows at M and N), sometimes at the vicinity of empty-looking spaces. C, capillary lumen. The length of the scale bar is indicated in each panel.
Fig 8. Representative microphotographs of different sites of the brain and spinal cord of l2hgdh -/- compared to l2hgdh +/+ mice.
A-D, transverse sections through the corpus callosum (cc); E-H, transverse sections through the hippocampus (dg, dentate gyrus); I-L, sections through the cerebellum and cerebellar nuclei (cn); M-P, transverse sections through the spinal cord (ah, anterior horn). Left panels (A, B, E, F, I, J, M, N) are representative of l2hgdh +/+ mice and right panels (C, D, G, H, K, L, O, P) of l2hgdh -/- mice. On each side, the right panel is a higher magnification of the left panel, where arrows indicate lesions seen in brains of l2hgdh -/- mice. Scale bars correspond to 300 μm or 50 μm as indicated.
Fig 9. Morris Water maze test.
The average escape latencies, (i.e., the time required for l2hgdh +/+ and l2hgdh -/- mice to reach the platform) on the training day and on the 3 consecutive trial days are shown in (A). The percentage of the time the mice spent in each quadrant of the platform arena on the 5th day of testing (once the platform is removed) is shown in (B). The average distance (in cm) between the position of the mice during the latter test and the position where the platform had been previously placed (Gallagher's coefficient) is shown in (C). Values are expressed as the means ± SEM (n = 9). Statistical analyses were performed using a two-way repeated measures ANOVA (A), a one-way ANOVA (B) followed by a Newman-Keuls test, or a Student t-test (C). *: p < 0.05.
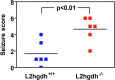
Fig 10. Sensitivity of l2hgdh -/- mice to the convulsant pentylenetetrazole.
Six l2hgdh +/+ and 6 l2hgdh -/- mice were placed in a plastic chamber (15 × 25 × 40 cm) and habituated for 10 min before intraperitoneal injection of a subconvulsive dose (40 mg/kg) of pentylenetetrazole (PTZ, Sigma-Aldrich, St. Louis, MO, USA). They were then video-tracked for 20 min and their behavior was classified and scored as follows: 0: normal; 1: immobilization; 2: facial, vibrissal and forelimb clonus (short myoclonic jerk); 3: myoclonic jerking consisting of a whole body jerk with or without irregular, bilateral forelimb movements; 4: generalized clonic seizures with kangaroo posture; 5: generalized tonic–clonic seizures with loss of posture tone; 6: death (Watanabe et al. 2013). Statistical analyses were performed using the Student t-test (p = 0.0062).
Similar articles
- L-2-hydroxyglutaric aciduria, a defect of metabolite repair.
Rzem R, Vincent MF, Van Schaftingen E, Veiga-da-Cunha M. Rzem R, et al. J Inherit Metab Dis. 2007 Oct;30(5):681-9. doi: 10.1007/s10545-007-0487-0. Epub 2007 Jun 21. J Inherit Metab Dis. 2007. PMID: 17603759 - [L-2-hydroxyglutaric aciduria, an error of metabolism].
Van Schaftingen E. Van Schaftingen E. Bull Mem Acad R Med Belg. 2007;162(10-12):451-6; discussion 456-7. Bull Mem Acad R Med Belg. 2007. PMID: 18557388 French. - L: -2-Hydroxyglutaric aciduria, a disorder of metabolite repair.
Van Schaftingen E, Rzem R, Veiga-da-Cunha M. Van Schaftingen E, et al. J Inherit Metab Dis. 2009 Apr;32(2):135-42. doi: 10.1007/s10545-008-1042-3. Epub 2008 Nov 21. J Inherit Metab Dis. 2009. PMID: 19020988 Review. - Structure and biochemical characterization of l-2-hydroxyglutarate dehydrogenase and its role in the pathogenesis of l-2-hydroxyglutaric aciduria.
Yang J, Chen X, Jin S, Ding J. Yang J, et al. J Biol Chem. 2024 Jan;300(1):105491. doi: 10.1016/j.jbc.2023.105491. Epub 2023 Nov 22. J Biol Chem. 2024. PMID: 37995940 Free PMC article. - A novel compound heterozygous mutation of the L2HGDH gene in a Chinese boy with L-2-hydroxyglutaric aciduria: case report and literature review.
Zhang Y, Wang C, Yang K, Wang S, Tian G, Chen Y. Zhang Y, et al. Neurol Sci. 2018 Oct;39(10):1697-1703. doi: 10.1007/s10072-018-3483-2. Epub 2018 Jul 6. Neurol Sci. 2018. PMID: 29980873 Review.
Cited by
- Saccharomyces cerevisiae Forms D-2-Hydroxyglutarate and Couples Its Degradation to D-Lactate Formation via a Cytosolic Transhydrogenase.
Becker-Kettern J, Paczia N, Conrotte JF, Kay DP, Guignard C, Jung PP, Linster CL. Becker-Kettern J, et al. J Biol Chem. 2016 Mar 18;291(12):6036-58. doi: 10.1074/jbc.M115.704494. Epub 2016 Jan 16. J Biol Chem. 2016. PMID: 26774271 Free PMC article. - Metabolism, Activity, and Targeting of D- and L-2-Hydroxyglutarates.
Ye D, Guan KL, Xiong Y. Ye D, et al. Trends Cancer. 2018 Feb;4(2):151-165. doi: 10.1016/j.trecan.2017.12.005. Epub 2018 Jan 5. Trends Cancer. 2018. PMID: 29458964 Free PMC article. Review. - The oncometabolite L-2-hydroxyglutarate is a common product of dipteran larval development.
Mahmoudzadeh NH, Fitt AJ, Schwab DB, Martenis WE, Nease LM, Owings CG, Brinkley GJ, Li H, Karty JA, Sudarshan S, Hardy RW, Moczek AP, Picard CJ, Tennessen JM. Mahmoudzadeh NH, et al. Insect Biochem Mol Biol. 2020 Dec;127:103493. doi: 10.1016/j.ibmb.2020.103493. Epub 2020 Nov 3. Insect Biochem Mol Biol. 2020. PMID: 33157229 Free PMC article. - Bioenergetics and translational metabolism: implications for genetics, physiology and precision medicine.
Hill BG, Shiva S, Ballinger S, Zhang J, Darley-Usmar VM. Hill BG, et al. Biol Chem. 2019 Dec 18;401(1):3-29. doi: 10.1515/hsz-2019-0268. Biol Chem. 2019. PMID: 31815377 Free PMC article. Review. - Drosophila larvae synthesize the putative oncometabolite L-2-hydroxyglutarate during normal developmental growth.
Li H, Chawla G, Hurlburt AJ, Sterrett MC, Zaslaver O, Cox J, Karty JA, Rosebrock AP, Caudy AA, Tennessen JM. Li H, et al. Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):1353-1358. doi: 10.1073/pnas.1614102114. Epub 2017 Jan 23. Proc Natl Acad Sci U S A. 2017. PMID: 28115720 Free PMC article.
References
- Duran M, Kamerling JP, Bakker HD, van Gennip AH, Wadman SK. L-2-Hydroxyglutaric aciduria: an inborn error of metabolism? J Inherit Metab Dis. 1980; 3: 109–112. - PubMed
- Chen E, Nyhan WL, Jakobs C, Greco CM, Barkovich AJ, Cox VA, et al. L-2-Hydroxyglutaric aciduria: neuropathological correlations and first report of severe neurodegenerative disease and neonatal death. J Inherit Metab Dis. 1996; 19: 335–343. - PubMed
- Topçu M, Aydin OF, Yalçinkaya C, Haliloğlu G, Aysun S, Anlar B, et al. L-2-hydroxyglutaric aciduria: a report of 29 patients. Turk J Pediatr. 2005; 47: 1–7. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
This work was supported by grants from the Fonds National de la Recherche Scientifique (FNRS) and the Interuniversity Attraction Poles Programme, Belgian Science Policy (Networks P7/43 and P7/13). Maria Veiga-da-Cunha is Chercheur Qualifié of the Belgian Fonds National de la Recherche Scientifique (FRS-FNRS). The Désordres Inflammatoires dans les Affections Neurologiques (DIANE) Centre of Excellence programme of the Région Wallonne. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials