Molecular chaperones and neuronal proteostasis - PubMed (original) (raw)
Review
Molecular chaperones and neuronal proteostasis
Heather L Smith et al. Semin Cell Dev Biol. 2015 Apr.
Abstract
Protein homeostasis (proteostasis) is essential for maintaining the functionality of the proteome. The disruption of proteostasis, due to genetic mutations or an age-related decline, leads to aberrantly folded proteins that typically lose their function. The accumulation of misfolded and aggregated protein is also cytotoxic and has been implicated in the pathogenesis of neurodegenerative diseases. Neurons have developed an intrinsic protein quality control network, of which molecular chaperones are an essential component. Molecular chaperones function to promote efficient folding and target misfolded proteins for refolding or degradation. Increasing molecular chaperone expression can suppress protein aggregation and toxicity in numerous models of neurodegenerative disease; therefore, molecular chaperones are considered exciting therapeutic targets. Furthermore, mutations in several chaperones cause inherited neurodegenerative diseases. In this review, we focus on the importance of molecular chaperones in neurodegenerative diseases, and discuss the advances in understanding their protective mechanisms.
Keywords: Heat shock response; Molecular chaperone; Neurodegeneration; Neuroprotection; Protein aggregation; Unfolded protein response.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
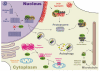
Fig. 1. HSJ1a acts restore proteostasis for several neurodegeneration proteins
Schematic showing the effect of HSJ1a on Htt, SOD1, parkin and tau in neurons. HSJ1a can bind to ubiquitylated oligomers of Htt in the nucleus blocking the recruitment of more misfolded Htt and further aggregation, leading to increases in soluble Htt and potential autophagic clearance of cytoplasmic Htt oligomers [39]. HSJ1 also facilitates proteasomal degradation of Htt [37]. HSJ1a blocks the aggregation of mutant parkin and stimulates its refolding, so that parkin can function in mitochondrial quality control [95]. In ALS, HSJ1a reduces the aggregation of mutant SOD1 and promotes the degradation by proteasome [45]. HSJ1a can bind tau, reduce tau phosphorylation and aggregation (Novoselov and Cheetham unpublished observations).
Similar articles
- Molecular chaperones biochemistry and role in neurodegenerative diseases.
Chaari A. Chaari A. Int J Biol Macromol. 2019 Jun 15;131:396-411. doi: 10.1016/j.ijbiomac.2019.02.148. Epub 2019 Mar 7. Int J Biol Macromol. 2019. PMID: 30853582 Review. - Challenging Proteostasis: Role of the Chaperone Network to Control Aggregation-Prone Proteins in Human Disease.
Sinnige T, Yu A, Morimoto RI. Sinnige T, et al. Adv Exp Med Biol. 2020;1243:53-68. doi: 10.1007/978-3-030-40204-4_4. Adv Exp Med Biol. 2020. PMID: 32297211 Free PMC article. Review. - Small Heat Shock Proteins: Protein Aggregation Amelioration and Neuro- and Age-Protective Roles.
Albinhassan TH, Alharbi BM, AlSuhaibani ES, Mohammad S, Malik SS. Albinhassan TH, et al. Int J Mol Sci. 2025 Feb 11;26(4):1525. doi: 10.3390/ijms26041525. Int J Mol Sci. 2025. PMID: 40003991 Free PMC article. Review. - Chaperones in Neurodegeneration.
Lindberg I, Shorter J, Wiseman RL, Chiti F, Dickey CA, McLean PJ. Lindberg I, et al. J Neurosci. 2015 Oct 14;35(41):13853-9. doi: 10.1523/JNEUROSCI.2600-15.2015. J Neurosci. 2015. PMID: 26468185 Free PMC article. Review. - Underlying mechanisms and chemical/biochemical therapeutic approaches to ameliorate protein misfolding neurodegenerative diseases.
Hekmatimoghaddam S, Zare-Khormizi MR, Pourrajab F. Hekmatimoghaddam S, et al. Biofactors. 2017 Nov;43(6):737-759. doi: 10.1002/biof.1264. Epub 2016 Feb 22. Biofactors. 2017. PMID: 26899445 Review.
Cited by
- HspB8 interacts with BAG3 in a "native-like" conformation forming a complex that displays chaperone-like activity.
Sciandrone B, Ami D, D'Urzo A, Angeli E, Relini A, Vanoni M, Natalello A, Regonesi ME. Sciandrone B, et al. Protein Sci. 2023 Jul;32(7):e4687. doi: 10.1002/pro.4687. Protein Sci. 2023. PMID: 37243950 Free PMC article. - Epigenetic inheritance of proteostasis and ageing.
Li C, Casanueva O. Li C, et al. Essays Biochem. 2016 Oct 15;60(2):191-202. doi: 10.1042/EBC20160025. Essays Biochem. 2016. PMID: 27744335 Free PMC article. Review. - Small Heat Shock Proteins, Big Impact on Protein Aggregation in Neurodegenerative Disease.
Webster JM, Darling AL, Uversky VN, Blair LJ. Webster JM, et al. Front Pharmacol. 2019 Sep 18;10:1047. doi: 10.3389/fphar.2019.01047. eCollection 2019. Front Pharmacol. 2019. PMID: 31619995 Free PMC article. Review. - Critical Roles of Dual-Specificity Phosphatases in Neuronal Proteostasis and Neurological Diseases.
Bhore N, Wang BJ, Chen YW, Liao YF. Bhore N, et al. Int J Mol Sci. 2017 Sep 13;18(9):1963. doi: 10.3390/ijms18091963. Int J Mol Sci. 2017. PMID: 28902166 Free PMC article. Review. - The Effect of Polyphenols on Protein Degradation Pathways: Implications for Neuroprotection.
Hajieva P. Hajieva P. Molecules. 2017 Jan 19;22(1):159. doi: 10.3390/molecules22010159. Molecules. 2017. PMID: 28106854 Free PMC article. Review.
References
- Ellis J. Proteins as molecular chaperones. Nature. 1987;328:378–379. - PubMed
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–333. - PubMed
- Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013;82:323–355. - PubMed
- Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Ann. Rev. Biochem. 2006;75:271–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical