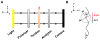Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage - PubMed (original) (raw)
Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage
Benjamin R Freedman et al. J Orthop Res. 2015 Jun.
Abstract
The complex structure of tendons relates to their mechanical properties. Previous research has associated the waviness of collagen fibers (crimp) during quasi-static tensile loading to tensile mechanical properties, but less is known about the role of fatigue loading on crimp properties. In this study (IACUC approved), mouse patellar tendons were fatigue loaded while an integrated plane polariscope simultaneously assessed crimp properties. We demonstrate a novel structural mechanism whereby tendon crimp amplitude and frequency are altered with fatigue loading. In particular, fatigue loading increased the crimp amplitude across the tendon width and length, and these structural alterations were shown to be both region and load dependent. The change in crimp amplitude was strongly correlated to mechanical tissue laxity (defined as the ratio of displacement and gauge length relative to the first cycle of fatigue loading assessed at constant load throughout testing), at all loads and regions evaluated. Together, this study highlights the role of fatigue loading on tendon crimp properties as a function of load applied and region evaluated, and offers an additional structural mechanism for mechanical alterations that may lead to ultimate tendon failure.
Keywords: collagen; imaging; ligament; patellar tendon; polarized light.
© 2015 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures
Figure 1. Experimental setup of a plane polariscope
The setup (A) consists of a backlight, two linear polarizers on either side of the tendon(s), and a camera. (B) The polarizer (P) and analyzer (A) are crossed at 90° and are oriented at the angle θ at which maximal extinction in the dark crimp bands occurred at preload.
Figure 2. Mechanical testing and image capture protocol
(A) Tendons were preloaded (a), preconditioned (b), imaged at three loads (0.1N, 0.5N, and 2.0N) (c), and fatigue loaded (d). After 10, 100, and 1000 cycle intervals of fatigue loading, images were captured at these three loads to quantify tendon crimp properties in the toe, transition, and linear regions of a representative load-displacement curve (B). This process was repeated until tendons reached 1000 fatigue loading cycles. (C) Four ROIs were selected representing the midsubstance (orange), insertion (yellow), center (solid), and lateral (dashed) regions of the tendon. ROIs were low pass filtered to enhance the visibility of light and dark bands and intensities were averaged across the ROI width (red dashed line) before being highpass filtered (blue line). From these spectra, the crimp amplitude and frequency were computed.
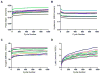
Figure 3. Effect of fatigue loading on tendon mechanical properties
Cycle number was a significant (p<0.001) factor for peak strain, tangent stiffness, hysteresis, and laxity. Individual lines indicate each specimen tested. With fatigue loading, peak strain, tangent stiffness, and laxity increased, whereas the hysteresis decreased.
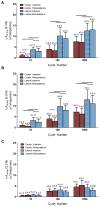
Figure 4
Δ Crimp amplitude (ΔAcrimp) increased with fatigue loading when assessed at (A) 0.1N (representative of the toe region of the force-displacement curve), (B) 0.5 N (representative of the transition region of the force-displacement curve), and (C) 2.0 N (representative of the linear region of the force-displacement curve). The ΔAcrimp demonstrated a load-dependent response, with lower values at higher loads. Bars indicate significant paired differences (p<0.0125) between the center and lateral ROIs and their corresponding insertion and midsubstances for a tendon after 10, 100, or 1000 cycles of fatigue loading. “u” indicates an intensity unit ranging between 1 and 256. *a,b,c,d indicates significant differences (p<0.0083) in the ROI when compared to 0, 10, 100, and 1000 cycles, respectively. “#” indicates trends (p<0.017).
Figure 5
Δ Crimp frequency (ΔFcrimp) decreased with fatigue loading when assessed at 0.1N. Bars indicate significant paired differences (p<0.0125) between the center and lateral ROIs and their corresponding insertion and midsubstances for a tendon after 10, 100, or 1000 cycles of fatigue loading. *a,b,c,d indicates significant differences (p<0.0083) in the ROI when compared to 0, 10, 100, and 1000 cycles, respectively. “#” indicates trends (p<0.017). Data for ΔFcrimp at 0.5 and 2.0N are not shown since the power of crimp frequencies decreases to near the power of noise (high frequencies) at high loads. This is an unavoidable trade off with our high resolution images that does not exist in the evaluation of the ΔAcrimp.
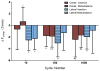
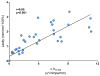
Figure 6
Tendon laxity (defined as the ratio of displacement from gauge length at a set threshold to the tissue displacement and displacement at a set threshold after the first cycle of fatigue loading) was strongly correlated to the change in crimp amplitude at 0.1N as assessed at 0, 10, 100, and 1000 cycles of fatigue life. This same relationship held at both higher loads (0.5N and 2.0N). “u” indicates an intensity unit ranging between 1 and 256.
Similar articles
- Tendon Biomechanics and Crimp Properties Following Fatigue Loading Are Influenced by Tendon Type and Age in Mice.
Zuskov A, Freedman BR, Gordon JA, Sarver JJ, Buckley MR, Soslowsky LJ. Zuskov A, et al. J Orthop Res. 2020 Jan;38(1):36-42. doi: 10.1002/jor.24407. Epub 2019 Jul 23. J Orthop Res. 2020. PMID: 31286548 Free PMC article. - Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury.
Freedman BR, Sarver JJ, Buckley MR, Voleti PB, Soslowsky LJ. Freedman BR, et al. J Biomech. 2014 Jun 27;47(9):2028-34. doi: 10.1016/j.jbiomech.2013.10.054. Epub 2013 Nov 11. J Biomech. 2014. PMID: 24280564 Free PMC article. - Inflammatory cells do not decrease the ultimate tensile strength of intact tendons in vivo and in vitro: protective role of mechanical loading.
Marsolais D, Duchesne E, Côté CH, Frenette J. Marsolais D, et al. J Appl Physiol (1985). 2007 Jan;102(1):11-7. doi: 10.1152/japplphysiol.00162.2006. Epub 2006 Aug 17. J Appl Physiol (1985). 2007. PMID: 16916923 - Fatigue loading of tendon.
Shepherd JH, Screen HR. Shepherd JH, et al. Int J Exp Pathol. 2013 Aug;94(4):260-70. doi: 10.1111/iep.12037. Int J Exp Pathol. 2013. PMID: 23837793 Free PMC article. Review. - In vivo investigation of tendon responses to mechanical loading.
Heinemeier KM, Kjaer M. Heinemeier KM, et al. J Musculoskelet Neuronal Interact. 2011 Jun;11(2):115-23. J Musculoskelet Neuronal Interact. 2011. PMID: 21625048 Review.
Cited by
- Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model.
Freedman BR, Gordon JA, Bhatt PR, Pardes AM, Thomas SJ, Sarver JJ, Riggin CN, Tucker JJ, Williams AW, Zanes RC, Hast MW, Farber DC, Silbernagel KG, Soslowsky LJ. Freedman BR, et al. J Orthop Res. 2016 Dec;34(12):2172-2180. doi: 10.1002/jor.23253. Epub 2016 Apr 13. J Orthop Res. 2016. PMID: 27038306 Free PMC article. - Accumulation of collagen molecular unfolding is the mechanism of cyclic fatigue damage and failure in collagenous tissues.
Zitnay JL, Jung GS, Lin AH, Qin Z, Li Y, Yu SM, Buehler MJ, Weiss JA. Zitnay JL, et al. Sci Adv. 2020 Aug 28;6(35):eaba2795. doi: 10.1126/sciadv.aba2795. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32923623 Free PMC article. - Tendinopathy and tendon material response to load: What we can learn from small animal studies.
Williamson PM, Freedman BR, Kwok N, Beeram I, Pennings J, Johnson J, Hamparian D, Cohen E, Galloway JL, Ramappa AJ, DeAngelis JP, Nazarian A. Williamson PM, et al. Acta Biomater. 2021 Oct 15;134:43-56. doi: 10.1016/j.actbio.2021.07.046. Epub 2021 Jul 27. Acta Biomater. 2021. PMID: 34325074 Free PMC article. Review. - Effects of immobilization angle on tendon healing after achilles rupture in a rat model.
Hillin CD, Fryhofer GW, Freedman BR, Choi DS, Weiss SN, Huegel J, Soslowsky LJ. Hillin CD, et al. J Orthop Res. 2019 Mar;37(3):562-573. doi: 10.1002/jor.24241. Epub 2019 Feb 28. J Orthop Res. 2019. PMID: 30720208 Free PMC article. - Postinjury biomechanics of Achilles tendon vary by sex and hormone status.
Fryhofer GW, Freedman BR, Hillin CD, Salka NS, Pardes AM, Weiss SN, Farber DC, Soslowsky LJ. Fryhofer GW, et al. J Appl Physiol (1985). 2016 Nov 1;121(5):1106-1114. doi: 10.1152/japplphysiol.00620.2016. Epub 2016 Sep 15. J Appl Physiol (1985). 2016. PMID: 27633741 Free PMC article.
References
- Diamant J, Keller A, Baer E, et al. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci. 1972;180:293–315. - PubMed
- Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical