Gut microbiota in multiple sclerosis: possible influence of immunomodulators - PubMed (original) (raw)
Gut microbiota in multiple sclerosis: possible influence of immunomodulators
Brandi L Cantarel et al. J Investig Med. 2015 Jun.
Abstract
Objectives: Differences in gut bacteria have been described in several autoimmune disorders. In this exploratory pilot study, we compared gut bacteria in patients with multiple sclerosis and healthy controls and evaluated the influence of glatiramer acetate and vitamin D treatment on the microbiota.
Methods: Subjects were otherwise healthy white women with or without relapsing-remitting multiple sclerosis who were vitamin D insufficient. Patients with multiple sclerosis were untreated or were receiving glatiramer acetate. Subjects collected stool at baseline and after 90 days of vitamin D3 (5000 IU/d) supplementation. The abundance of operational taxonomic units was evaluated by hybridization of 16S rRNA to a DNA microarray.
Results: While there was overlap of gut bacterial communities, the abundance of some operational taxonomic units, including Faecalibacterium, was lower in patients with multiple sclerosis. Glatiramer acetate-treated patients with multiple sclerosis showed differences in community composition compared with untreated subjects, including Bacteroidaceae, Faecalibacterium, Ruminococcus, Lactobacillaceae, Clostridium, and other Clostridiales. Compared with the other groups, untreated patients with multiple sclerosis had an increase in the Akkermansia, Faecalibacterium, and Coprococcus genera after vitamin D supplementation.
Conclusions: While overall bacterial communities were similar, specific operational taxonomic units differed between healthy controls and patients with multiple sclerosis. Glatiramer acetate and vitamin D supplementation were associated with differences or changes in the microbiota. This study was exploratory, and larger studies are needed to confirm these preliminary results.
Figures
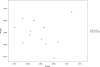
Figure 1
These samples were not available (n=2) or not used (n=1; sample given late) for the pre- vs post-vitamin D supplementation analysis. MS= Multiple sclerosis HC= Healthy control GA= glatiramer acetate *One GA-treated subject (who didn't contribute a pre-vitamin D supplementation sample) took antibiotics for an infection prior to the study (azithromycin for sinus infection), and ** one baseline sample was inadvertently stored at −20° C for several days before being moved to −80° C. There was no apparent impact on results of either affected sample (i.e. they did not appear to be outliers).
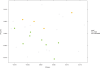
Figure 2
PCoA by phenotype and supplementation. Principal component analysis is a transformation of Weighted Unifrac distance, a pair-wise distance between samples based the calculation of the shared branches a the phylogenetic tree of the representative rRNA genes from 19757 operational taxonomic units present in at least one sample, weighted by abundance. HC, healthy control; MS, affected; pre/post, refers to supplementation with Vitamin D. Axis 1: 33% of variation explained. Axis 2: 21% of variation explained. All samples were used to generate the plots; for plot clarity, the excluded sample (late sample from one MS subject) was removed from this figure (see Supplementary Figure 1 for unaltered plot).
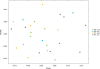
Figure 3
Differential clustering based on glatiramer acetate treatment. Principal component analysis is a transformation of Weighted Unifrac distance, a pair-wise distance between samples based the calculation of the shared branches a the phylogenetic tree of the representative rRNA genes from 19757 operational taxonomic units present in at least one sample, weighted by abundance. MS, affected where patients are either on GA or untreated. Axis 1: 33% of variation explained. Axis 2: 21% of variation explained. All samples were used to generate the plots; for plot clarity, the excluded samples (HCs) were removed from this figure (see Supplementary Figure 2 for unaltered plot).
Figure 4
Distribution of change of overall microbial abundance after vitamin D supplementation. Change in relative microbial OTU abundance in fecal samples between pre- and post- vitamin D supplementation samples in each subject group using only OTUs present in both samples. The “*” signifies a significant change in the distribution between HC and untreated.
Figure 5
Distribution of statistically significant changes of OTU abundance in taxa. Change in relative abundance of taxa between the pre- and post- Vitamin D supplement time points where statistically significant changes between (A) untreated MS and healthy control or treated MS and (B) treated MS and untreated MS or healthy control (HC). The “*” signifies a significant change in the distribution between HC and untreated.
Similar articles
- Disease-modifying therapies alter gut microbial composition in MS.
Katz Sand I, Zhu Y, Ntranos A, Clemente JC, Cekanaviciute E, Brandstadter R, Crabtree-Hartman E, Singh S, Bencosme Y, Debelius J, Knight R, Cree BAC, Baranzini SE, Casaccia P. Katz Sand I, et al. Neurol Neuroimmunol Neuroinflamm. 2018 Oct 26;6(1):e517. doi: 10.1212/NXI.0000000000000517. eCollection 2019 Jan. Neurol Neuroimmunol Neuroinflamm. 2018. PMID: 30568995 Free PMC article. - Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis.
Iaffaldano P, Lucisano G, Pozzilli C, Brescia Morra V, Ghezzi A, Millefiorini E, Patti F, Lugaresi A, Zimatore GB, Marrosu MG, Amato MP, Bertolotto A, Bergamaschi R, Granella F, Coniglio G, Tedeschi G, Sola P, Lus G, Ferrò MT, Iuliano G, Corea F, Protti A, Cavalla P, Guareschi A, Rodegher M, Paolicelli D, Tortorella C, Lepore V, Prosperini L, Saccà F, Baroncini D, Comi G, Trojano M; Italian iMed-Web database. Iaffaldano P, et al. Brain. 2015 Nov;138(Pt 11):3275-86. doi: 10.1093/brain/awv260. Epub 2015 Sep 11. Brain. 2015. PMID: 26362907 - Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 Multiple Sclerosis Study Group.
Johnson KP, Brooks BR, Ford CC, Goodman A, Guarnaccia J, Lisak RP, Myers LW, Panitch HS, Pruitt A, Rose JW, Kachuck N, Wolinsky JS. Johnson KP, et al. Mult Scler. 2000 Aug;6(4):255-66. doi: 10.1177/135245850000600407. Mult Scler. 2000. PMID: 10962546 Clinical Trial. - [Glatiramer acetate (Copaxone) influence on different stages of multiple sclerosis pathogenesis].
Shmidt TE, Zhuchenko TD, Iakhno NN. Shmidt TE, et al. Zh Nevrol Psikhiatr Im S S Korsakova. 2003;(Spec No 2):79-82. Zh Nevrol Psikhiatr Im S S Korsakova. 2003. PMID: 12938640 Review. Russian. - [A comparative evaluation of immunomodulating drugs in the treatment of remitting multiple sclerosis].
Zvartau ME, Kaon K, Lisak RF, Kan OA, Skoromets AA. Zvartau ME, et al. Zh Nevrol Psikhiatr Im S S Korsakova. 2004;104(7):66-71. Zh Nevrol Psikhiatr Im S S Korsakova. 2004. PMID: 15347038 Review. Russian. No abstract available.
Cited by
- Using data science for medical decision making case: role of gut microbiome in multiple sclerosis.
Hasic Telalovic J, Music A. Hasic Telalovic J, et al. BMC Med Inform Decis Mak. 2020 Oct 12;20(1):262. doi: 10.1186/s12911-020-01263-2. BMC Med Inform Decis Mak. 2020. PMID: 33046051 Free PMC article. - Gut Dysbiosis and Dietary Interventions in Rheumatoid Arthritis-A Narrative Review.
Bakinowska E, Stańska W, Kiełbowski K, Szwedkowicz A, Boboryko D, Pawlik A. Bakinowska E, et al. Nutrients. 2024 Sep 23;16(18):3215. doi: 10.3390/nu16183215. Nutrients. 2024. PMID: 39339815 Free PMC article. Review. - Induction of Regulatory Properties in the Intestinal Immune System by Dimethyl Fumarate in Lewis Rat Experimental Autoimmune Neuritis.
Pitarokoili K, Bachir H, Sgodzai M, Grüter T, Haupeltshofer S, Duscha A, Pedreiturria X, Motte J, Gold R. Pitarokoili K, et al. Front Immunol. 2019 Sep 10;10:2132. doi: 10.3389/fimmu.2019.02132. eCollection 2019. Front Immunol. 2019. PMID: 31552056 Free PMC article. - Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course.
Ventura RE, Iizumi T, Battaglia T, Liu M, Perez-Perez GI, Herbert J, Blaser MJ. Ventura RE, et al. Sci Rep. 2019 Nov 8;9(1):16396. doi: 10.1038/s41598-019-52894-z. Sci Rep. 2019. PMID: 31705027 Free PMC article. - The Combined Beneficial Effects of Postbiotic Butyrate on Active Vitamin D3-Orchestrated Innate Immunity to Salmonella Colitis.
Huang FC, Huang SC. Huang FC, et al. Biomedicines. 2021 Sep 22;9(10):1296. doi: 10.3390/biomedicines9101296. Biomedicines. 2021. PMID: 34680413 Free PMC article.
References
- Granieri E, Casetta I, Govoni V, et al. The increasing incidence and prevalence of MS in a Sardinian province. Neurology. 2000;55:842–848. - PubMed
- Stanghellini V, Barbara G, Cremon C, et al. Gut microbiota and related diseases: clinical features. Intern Emerg Med. 2010;5:S57–S63. - PubMed
- Ochoa-Reparaz J, Mielcarz DW, Begum-Haque S, Kasper LH. Gut, bugs, and brain: role of commensal bacteria in the control of central nervous system disease. Ann Neurol. 2011;69:240–247. - PubMed
- Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. - PubMed
- Hazen TC, Dubinsky EA, DeSantis TZ, et al. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science. 2010;330:204–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources