Avoidance of autophagy mediated by PlcA or ActA is required for Listeria monocytogenes growth in macrophages - PubMed (original) (raw)
Avoidance of autophagy mediated by PlcA or ActA is required for Listeria monocytogenes growth in macrophages
Gabriel Mitchell et al. Infect Immun. 2015 May.
Abstract
Listeria monocytogenes is a facultative intracellular pathogen that escapes from phagosomes and grows in the cytosol of infected host cells. Most of the determinants that govern its intracellular life cycle are controlled by the transcription factor PrfA, including the pore-forming cytolysin listeriolysin O (LLO), two phospholipases C (PlcA and PlcB), and ActA. We constructed a strain that lacked PrfA but expressed LLO from a PrfA-independent promoter, thereby allowing the bacteria to gain access to the host cytosol. This strain did not grow efficiently in wild-type macrophages but grew normally in macrophages that lacked ATG5, a component of the autophagy LC3 conjugation system. This strain colocalized more with the autophagy marker LC3 (42% ± 7%) at 2 h postinfection, which constituted a 5-fold increase over the colocalization exhibited by the wild-type strain (8% ± 6%). While mutants lacking the PrfA-dependent virulence factor PlcA, PlcB, or ActA grew normally, a double mutant lacking both PlcA and ActA failed to grow in wild-type macrophages and colocalized more with LC3 (38% ± 5%). Coexpression of LLO and PlcA in a PrfA-negative strain was sufficient to restore intracellular growth and decrease the colocalization of the bacteria with LC3. In a cell-free assay, purified PlcA protein blocked LC3 lipidation, a key step in early autophagosome biogenesis, presumably by preventing the formation of phosphatidylinositol 3-phosphate (PI3P). The results of this study showed that avoidance of autophagy by L. monocytogenes primarily involves PlcA and ActA and that either one of these factors must be present for L. monocytogenes growth in macrophages.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
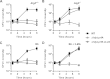
FIG 1
Intracellular growth of a Δ_prfA_ strain expressing LLO. Kinetics of intracellular growth for wild-type, Δ_hly_ Δ_prfA_ (with the empty integrated vector pHpPL3), and Δ_hly_ Δ_prfA_ cLLO in Atg5+/+ BMDM (A), _Atg5_−/− BMDM (B), B6 BMDM (C), and B6 BMDM exposed to 3-MA (D) are shown. Results are expressed as means and standard deviations obtained from at least 3 independent experiments.
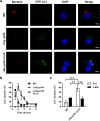
FIG 2
Colocalization of LC3 with a Δ_prfA_ strain expressing LLO. (A) Representative micrographs of GFP-LC3 BMDM infected for 2 h with 10403S, Δ_hly_ Δ_prfA_, and Δ_hly_ Δ_prfA_ cLLO. Infected cells were stained for L. monocytogenes (red), GFP-LC3 (green), and DNA (blue). (B) Colocalization kinetics of GFP-LC3 with WT, Δ_hly_ Δ_prfA_ (pHpPL3), and Δ_hly_ Δ_prfA_ cLLO. Proportions of GFP-LC3+ bacteria are expressed as a percentage of total intracellular L. monocytogenes. The Δ_hly_ Δ_prfA_ cLLO strain showed increased colocalization with LC3 in comparison to the WT strain from 2 to 4 h postinfection (P < 0.0001 for each time points; two-way ANOVA with Dunnett's posttest). (C) Effect of 3-MA on the colocalization of GFP-LC3 with WT and Δ_hly_ Δ_prfA_ cLLO at 2 h postinfection. Relevant statistically significant differences are indicated (**, P < 0.01; ***, P < 0.001 [ANOVA with Tukey's posttest]). Results are expressed as means and standard deviations obtained from at least 3 independent experiments. Bars = 5 μm.
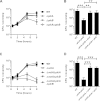
FIG 3
Intracellular growth of Δ_actA_, Δ_plcA_, and Δ_plcB_ strains. (A) Kinetics of intracellular growth for WT, Δ_plcA_, Δ_plcB_, and Δ_plcA_ Δ_plcB_ strains in BMDM. (B) CFU recovered from BMDM infected with WT, Δ_plcA_ Δ_plcB_, Δ_plcA_ Δ_plcB_ pPL2-plcA, and Δ_plcA_ Δ_plcB_ pPL2-P_actA_-plcB organisms for 8 h. Statistically significant differences between strains are indicated (**, P < 0.01; ***, P < 0.001 [one-way ANOVA with Tukey's posttest]). (C) Kinetics of intracellular growth for WT, Δ_actA_, Δ_actA_ Δ_plcA_, Δ_actA_ Δ_plcB_ and Δ_actA_ Δ_plcA_ Δ_plcB_ strains in BMDM. (D) CFU recovered from BMDM infected with WT, Δ_actA_ Δ_plcA_, Δ_actA_ Δ_plcA_ pPL2-actA, and Δ_actA_ Δ_plcA_ pPL2-plcA strains for 8 h. Statistically significant differences between strains are indicated (***, P < 0.001 [one-way ANOVA with Tukey's posttest]). Results are expressed as means and standard deviations obtained from at least 3 independent experiments.
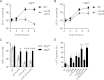
FIG 4
Intracellular growth of Δ_actA_, Δ_plcA_, and Δ_plcB_ strains in Atg5_−/− BMDM and colocalization with LC3. Kinetics of intracellular growth for WT and Δ_actA Δ_plcA_ strains in Atg5+/+ (A) and Atg5_−/− (B) BMDM are shown. (C) CFU recovered from Atg5+/+ and Atg5_−/− BMDM infected with WT, Δ_plcA Δ_plcB, Δ_actA_ Δ_plcA_, and Δ_actA_ Δ_plcA ΔplcB_ strains for 8 h. Statistically significant differences between Atg5+/+ and Atg5_−/− BMDM are indicated for each strain (*, P < 0.05; ***, P < 0.001; unpaired t test). (D) Colocalization of GFP-LC3 with WT, Δ_hly, Δ_plcA_, Δ_plcB_, Δ_plcA_ Δ_plcB_, Δ_actA_, Δ_actA_ Δ_plcA_, Δ_actA_ Δ_plcB_, and Δ_actA_ Δ_plcA_ Δ_plcB_ strains at 2 h postinfection. Proportions of GFP-LC3+ bacteria are expressed as a percentage of total intracellular L. monocytogenes. Statistically significant differences in comparison to WT, Δ_actA_ and Δ_plcA_ strains are indicated by the letters a, b, and c, respectively (P < 0.05 [one-way ANOVA with Tukey's posttest]). Results are expressed as means and standard deviations obtained from at least 3 independent experiments.
FIG 5
Intracellular growth and colocalization with LC3 of a Δ_prfA_ strain expressing LLO and PlcA. (A) Kinetic of intracellular growth for WT, Δ_hly_ Δ_prfA_ cLLO, and Δ_hly_ Δ_prfA_ cLLO cPlcA strains in BMDM. (B) Quantification of GFP-LC3+ bacteria for WT, Δ_hly_ Δ_prfA_ cLLO, and Δ_hly_ Δ_prfA_ cLLO cPlcA strains expressed as a percentage of total intracellular L. monocytogenes at 2 h postinfection. Significant differences between strains are indicated (**, P < 0.01; ***, P < 0.001 [one-way ANOVA with Tukey's posttest]). Results are expressed as means and standard deviations obtained from at least 3 independent experiments.
FIG 6
Effect of PlcA and PlcB on in vitro LC3 lipidation, membrane integrity, and PI3P levels. The membrane fraction was digested with the indicated concentrations of PlcA and PlcA(W49A) (A) or PlcB and PlcB(D55N) (B). The postdigestion membranes were then collected and subjected to in vitro LC3 lipidation assay and PI3P measurement followed by immunoblotting with the indicated antibodies. Membrane integrity was evaluated by measuring the levels of the intraluminal protein disulfide isomerase (PDI) in the membrane fraction. ERGIC-53 is the membrane loading control. Membrane levels of VPS34 were also evaluated.
Similar articles
- Listeria monocytogenes triggers noncanonical autophagy upon phagocytosis, but avoids subsequent growth-restricting xenophagy.
Mitchell G, Cheng MI, Chen C, Nguyen BN, Whiteley AT, Kianian S, Cox JS, Green DR, McDonald KL, Portnoy DA. Mitchell G, et al. Proc Natl Acad Sci U S A. 2018 Jan 9;115(2):E210-E217. doi: 10.1073/pnas.1716055115. Epub 2017 Dec 26. Proc Natl Acad Sci U S A. 2018. PMID: 29279409 Free PMC article. - Transcriptional regulation of prfA and PrfA-regulated virulence genes in Listeria monocytogenes.
Bohne J, Sokolovic Z, Goebel W. Bohne J, et al. Mol Microbiol. 1994 Mar;11(6):1141-50. doi: 10.1111/j.1365-2958.1994.tb00390.x. Mol Microbiol. 1994. PMID: 8022283 - Autophagy in immunity against intracellular bacteria.
Huang J, Brumell JH. Huang J, et al. Curr Top Microbiol Immunol. 2009;335:189-215. doi: 10.1007/978-3-642-00302-8_9. Curr Top Microbiol Immunol. 2009. PMID: 19802566 Review. - ActA of Listeria monocytogenes and Its Manifold Activities as an Important Listerial Virulence Factor.
Pillich H, Puri M, Chakraborty T. Pillich H, et al. Curr Top Microbiol Immunol. 2017;399:113-132. doi: 10.1007/82_2016_30. Curr Top Microbiol Immunol. 2017. PMID: 27726006 Review.
Cited by
- A glycine-rich PE_PGRS protein governs mycobacterial actin-based motility.
Hill NS, Welch MD. Hill NS, et al. Nat Commun. 2022 Jun 24;13(1):3608. doi: 10.1038/s41467-022-31333-0. Nat Commun. 2022. PMID: 35750685 Free PMC article. - Bacterial Pathogens versus Autophagy: Implications for Therapeutic Interventions.
Kimmey JM, Stallings CL. Kimmey JM, et al. Trends Mol Med. 2016 Dec;22(12):1060-1076. doi: 10.1016/j.molmed.2016.10.008. Epub 2016 Nov 17. Trends Mol Med. 2016. PMID: 27866924 Free PMC article. Review. - Emerging Evasion Mechanisms of Macrophage Defenses by Pathogenic Bacteria.
Leseigneur C, Lê-Bury P, Pizarro-Cerdá J, Dussurget O. Leseigneur C, et al. Front Cell Infect Microbiol. 2020 Sep 25;10:577559. doi: 10.3389/fcimb.2020.577559. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33102257 Free PMC article. Review. - The Role of Autophagy and Autophagy Receptor NDP52 in Microbial Infections.
Fan S, Wu K, Zhao M, Zhu E, Ma S, Chen Y, Ding H, Yi L, Zhao M, Chen J. Fan S, et al. Int J Mol Sci. 2020 Mar 16;21(6):2008. doi: 10.3390/ijms21062008. Int J Mol Sci. 2020. PMID: 32187990 Free PMC article. Review. - Recombinant broad-range phospholipase C from Listeria monocytogenes exhibits optimal activity at acidic pH.
Huang Q, Gershenson A, Roberts MF. Huang Q, et al. Biochim Biophys Acta. 2016 Jun;1864(6):697-705. doi: 10.1016/j.bbapap.2016.03.008. Epub 2016 Mar 11. Biochim Biophys Acta. 2016. PMID: 26976751 Free PMC article.
References
- Goldfine H, Bannam T, Johnston NC, Zuckert WR. 1998. Bacterial phospholipases and intracellular growth: the two distinct phospholipases C of Listeria monocytogenes. Symp Ser Soc Appl Microbiol 27:7S–14S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1P01AI63302/AI/NIAID NIH HHS/United States
- 1R01 AI27655/AI/NIAID NIH HHS/United States
- R01 AI027655/AI/NIAID NIH HHS/United States
- T32 AI100829/AI/NIAID NIH HHS/United States
- P01 AI063302/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources