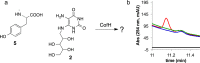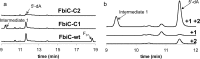Biosynthetic versatility and coordinated action of 5'-deoxyadenosyl radicals in deazaflavin biosynthesis - PubMed (original) (raw)
. 2015 Apr 29;137(16):5406-13.
doi: 10.1021/ja513287k. Epub 2015 Apr 20.
Affiliations
- PMID: 25781338
- PMCID: PMC4416281
- DOI: 10.1021/ja513287k
Biosynthetic versatility and coordinated action of 5'-deoxyadenosyl radicals in deazaflavin biosynthesis
Benjamin Philmus et al. J Am Chem Soc. 2015.
Abstract
Coenzyme F420 is a redox cofactor found in methanogens and in various actinobacteria. Despite the major biological importance of this cofactor, the biosynthesis of its deazaflavin core (8-hydroxy-5-deazaflavin, F(o)) is still poorly understood. F(o) synthase, the enzyme involved, is an unusual multidomain radical SAM enzyme that uses two separate 5'-deoxyadenosyl radicals to catalyze F(o) formation. In this paper, we report a detailed mechanistic study on this complex enzyme that led us to identify (1) the hydrogen atoms abstracted from the substrate by the two radical SAM domains, (2) the second tyrosine-derived product, (3) the reaction product of the CofH-catalyzed reaction, (4) the demonstration that this product is a substrate for CofG, and (5) a stereochemical study that is consistent with the formation of a p-hydroxybenzyl radical at the CofH active site. These results enable us to propose a mechanism for F(o) synthase and uncover a new catalytic motif in radical SAM enzymology involving the use of two 5'-deoxyadenosyl radicals to mediate the formation of a complex heterocycle.
Figures
Figure 1
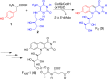
Biosynthesis of the deazaflavin chromophore of F420 (Fo, 3). The structure of F420 shown contains a single glutamic acid, which is designated as F420-1. The number of glutamic acid residues varies.
Figure 2
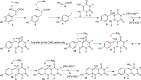
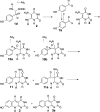
Mechanistic proposal for the Fo-synthase-catalyzed reaction. AdG and AdH correspond to the 5′-deoxyadenosyl radicals generated at the CofG and the CofH active sites by reduction of SAM using the active site [4Fe-4S]+1 cluster., Abstraction of the tyrosine amine hydrogen atom by the CofH 5′-deoxyadenosyl radical gives 5, which then undergoes fragmentation leading to the formation of the _p_-hydroxybenzyl radical 7. This then undergoes addition to the double bond of diaminouracil 2 followed by oxidation to give 9. Transfer of 9 from the CofH active site to the CofG active site gives 10. Hydrogen atom abstraction, by the CofG 5′-deoxyadenosyl radical, gives 11. Cyclization to 13, followed by oxidation by the [4Fe-4S]+2 cluster and elimination of ammonia completes the formation of the deazaflavin 3.
Figure 3
Deuterium incorporation into 5′-deoxyadenosine (left spectrum, [M + H]+ = 252.1 Da) and Fo (right spectrum, [M + H]+ = 364.1 Da, [M + K]+ = 402.1 Da) from various isotopologues of tyrosine: (a) tyrosine, (b) D7-tyrosine, or (c) 3,3-D2-tyrosine. The percentages written above the 253.1 Da peak indicate the relative height compared to the 252.1 peak. Each reaction mixture contained methyl viologen, SAM, diaminouracil, D_n_-tyrosine, CofH, and CofG.
Figure 4
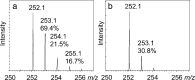
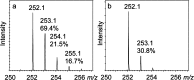
CofH-catalyzed deuterium incorporation into 5′-deoxyadenosine in reactions run in 80% D2O containing SAM, diaminouracil and methyl viologen. (a) Reaction run in the presence of tyrosine showing an enhanced 253.1 peak (calculated to be 69.4, 21.5 and 16.7% for the [M + D + H]+, [M + D2 + H]+, [M + D3 + H]+, respectively, in panel a and 30.8% for the [M + D + H]+ in panel b). (b) Reaction run in the absence of tyrosine showing the uncoupled production of 5′-deoxyadenosine. 5′-deoxyadenosine has an expected [M + H]+ of 252.1 m/z with the 253.1 peak calculated to be 12.9 ± 3%.
Figure 5
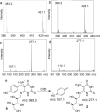
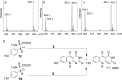
Trapping of the tyrosine-derived glyoxylate 15. (a) Proposed scheme for the generation of glyoxylate 15 by hydrolysis of the glycine imine 6 and its trapping using _o_-phenylenediamine 16. (b) Extracted ion chromatograms at m/z 148.05 ± 0.1 of labeled 2-quinoxalinol 17 generated from a reaction mixture containing CofG + CofH + SAM + sodium dithionite + [15N,13C9]-tyrosine + 2 (red trace); CofH + SAM + sodium dithionite + [15N,13C9]-tyrosine + 2 (blue trace). Control reactions lacking SAM, sodium dithionite, tyrosine, or CofH did not produce labeled glyoxylate.
Figure 6
Detection of the CofH reaction product. (a) Reaction catalyzed by CofH. (b) HPLC chromatogram of the reaction mixture containing CofH + SAM + methyl viologen (reduced) + 1 + 2 (red) showing a new product eluting after 11.15 min. Reaction mixtures lacking reduced methyl viologen (green), SAM (black), or tyrosine (blue) did not show this product.
Figure 7
MS analysis of the CofH reaction product. (a) Mass spectrum of the product generated from tyrosine ([M + H]+ obs. 383.1573, calcd for 9, 383.1561, 3.3 ppm error, [M + K]+ obs. 421.1132, calcd for 9, 421.1120, 2.8 ppm error; (b) mass spectrum of the product generated from [15N,13C9]-Tyr ([M + H]+ obs. 390.1775, calcd for [13C7]-9, 390.1796, 5.4 ppm error, [M + K]+ obs. 428.1322, calcd for 9, 428.1355, 7.7 ppm error); (c) MS2 of the product from tyrosine (m/z 383.1); (d) MS2 of the product from [15N,13C9]-tyrosine (m/z 390.1). The isolation width was 10 m/z and the collision energy was 30 V. (e) Proposed CID fragmentation pattern of the CofH product 9.
Figure 8
Incorporation of deuterium into 5′-deoxyadenosine in reactions consisting of SAM, [5,5-D2]-9 and dithionite reduced CofG. (a) Mass spectrum of 5′-deoxyadenosine (calcd 252.1 with a 253.1 peak calculated to be 12.9 ± 3% relative intensity; (b) mass spectrum of Fo (calcd 364.1). The presence of the 252.1 peak in the 5′-deoxyadenosine MS is most likely due to the uncoupled production of 5′-deoxyadenosine.
Figure 9
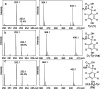
Detection of an intermediate produced by FbiC-C1. (a) HPLC analysis (Abs 257 nm) of the reaction mixtures containing SAM + tyrosine (1) + diaminouracil (2) + flavodoxin/flavodoxin reductase in the presence of FbiC (wild-type) (25 μM) or variants (FbiC-C1 or FbiC-C2, each at 50 μM). (b) HPLC analysis (Abs 257 nm) of the reaction of FbiC-C1 demonstrating that the production of the intermediate is dependent on both tyrosine (1) and diaminouracil (2).
Figure 10
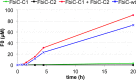
Fo production by FbiC-C1 and FbiC-C2 variants compared to FbiC-wt. The FbiC proteins were incubated with SAM, tyrosine (1) and diaminouracil (2), and the reaction was initiated with the flavodoxin/flavodoxin reductase system. The estimated rate of FbiC-catalyzed Fo formation is 2.4 × 10–5 s–1.
Figure 11
Stereochemistry of deuterium transfer from C3 of tyrosine during CofG/CofH-catalyzed Fo formation. (a) Mass spectrum of Fo derived from [3,3-D2]-Tyr; (b) mass spectrum of Fo derived from [2,3-D2, 3_S_]-Tyr (1c); (c) mass spectrum of Fo derived from [3-D, 3_R_]-Tyr (1d). (Fo calcd [MH + H]+ 364.1, calcd [MD + H]+ 365.1, calcd [MH + K]+ 402.1, calcd [MD + K]+ 403.1.
Figure 12
Comparison of the reaction catalyzed by ThiH (thiamin biosynthesis) HydG (FeFe hydrogenase biosynthesis), NosL (Nosiheptide biosynthesis), and the proposed reaction catalyzed by CofH (F420 biosynthesis). Reversible abstraction of the exchangeable hydrogen atom has been observed for ThiH, (Begley,T. P. and Mehta A, unpublished) HydG, and CofH and structural studies on NosL clearly demonstrates abstraction of the amino hydrogen.
Figure 13
Strategy to detect β bond cleavage involving loss of stereochemical information at C3 of tyrosine.
Similar articles
- Biosynthesis of F0, precursor of the F420 cofactor, requires a unique two radical-SAM domain enzyme and tyrosine as substrate.
Decamps L, Philmus B, Benjdia A, White R, Begley TP, Berteau O. Decamps L, et al. J Am Chem Soc. 2012 Nov 7;134(44):18173-6. doi: 10.1021/ja307762b. Epub 2012 Oct 24. J Am Chem Soc. 2012. PMID: 23072415 - Identification of the 7,8-didemethyl-8-hydroxy-5-deazariboflavin synthase required for coenzyme F(420) biosynthesis.
Graham DE, Xu H, White RH. Graham DE, et al. Arch Microbiol. 2003 Dec;180(6):455-64. doi: 10.1007/s00203-003-0614-8. Epub 2003 Oct 31. Arch Microbiol. 2003. PMID: 14593448 - Radical SAM enzymes involved in the biosynthesis of purine-based natural products.
Bandarian V. Bandarian V. Biochim Biophys Acta. 2012 Nov;1824(11):1245-53. doi: 10.1016/j.bbapap.2012.07.014. Epub 2012 Aug 3. Biochim Biophys Acta. 2012. PMID: 22902275 Free PMC article. Review. - Aminofutalosine Synthase (MqnE): A New Catalytic Motif in Radical SAM Enzymology.
Joshi S, Fedoseyenko D, Mahanta N, Begley TP. Joshi S, et al. Methods Enzymol. 2018;606:179-198. doi: 10.1016/bs.mie.2018.05.002. Epub 2018 Jul 18. Methods Enzymol. 2018. PMID: 30097092 - Biosynthesis of vitamin B2: Structure and mechanism of riboflavin synthase.
Fischer M, Bacher A. Fischer M, et al. Arch Biochem Biophys. 2008 Jun 15;474(2):252-65. doi: 10.1016/j.abb.2008.02.008. Epub 2008 Feb 14. Arch Biochem Biophys. 2008. PMID: 18298940 Review.
Cited by
- Cofactor F420: an expanded view of its distribution, biosynthesis and roles in bacteria and archaea.
Grinter R, Greening C. Grinter R, et al. FEMS Microbiol Rev. 2021 Sep 8;45(5):fuab021. doi: 10.1093/femsre/fuab021. FEMS Microbiol Rev. 2021. PMID: 33851978 Free PMC article. - Microbial Pigments: Major Groups and Industrial Applications.
Barreto JVO, Casanova LM, Junior AN, Reis-Mansur MCPP, Vermelho AB. Barreto JVO, et al. Microorganisms. 2023 Dec 4;11(12):2920. doi: 10.3390/microorganisms11122920. Microorganisms. 2023. PMID: 38138065 Free PMC article. Review. - Highlighting the Unique Roles of Radical _S_-Adenosylmethionine Enzymes in Methanogenic Archaea.
Boswinkle K, McKinney J, Allen KD. Boswinkle K, et al. J Bacteriol. 2022 Aug 16;204(8):e0019722. doi: 10.1128/jb.00197-22. Epub 2022 Jul 26. J Bacteriol. 2022. PMID: 35880875 Free PMC article. Review. - Physiology, Biochemistry, and Applications of F420- and Fo-Dependent Redox Reactions.
Greening C, Ahmed FH, Mohamed AE, Lee BM, Pandey G, Warden AC, Scott C, Oakeshott JG, Taylor MC, Jackson CJ. Greening C, et al. Microbiol Mol Biol Rev. 2016 Apr 27;80(2):451-93. doi: 10.1128/MMBR.00070-15. Print 2016 Jun. Microbiol Mol Biol Rev. 2016. PMID: 27122598 Free PMC article. Review. - Biosynthesis of the sactipeptide Ruminococcin C by the human microbiome: Mechanistic insights into thioether bond formation by radical SAM enzymes.
Balty C, Guillot A, Fradale L, Brewee C, Lefranc B, Herrero C, Sandström C, Leprince J, Berteau O, Benjdia A. Balty C, et al. J Biol Chem. 2020 Dec 4;295(49):16665-16677. doi: 10.1074/jbc.RA120.015371. Epub 2020 Sep 24. J Biol Chem. 2020. PMID: 32972973 Free PMC article.
References
- Kiener A.; Husain I.; Sancar A.; Walsh C. J. Biol. Chem. 1989, 264, 13880. - PubMed
- Malhotra K.; Kim S. T.; Walsh C.; Sancar A. J. Biol. Chem. 1992, 267, 15406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DK044083/DK/NIDDK NIH HHS/United States
- R56 DK044083/DK/NIDDK NIH HHS/United States
- DK44083/DK/NIDDK NIH HHS/United States
- 617053/ERC_/European Research Council/International
- R01 DK044083/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases