Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis - PubMed (original) (raw)
. 2015 Mar 18;10(3):e0121246.
doi: 10.1371/journal.pone.0121246. eCollection 2015.
Jin Woo Song 2, Sarah G Chu 1, Kenji Mizumura 3, Juan C Osorio 1, Ying Shi 1, Souheil El-Chemaly 1, Chun Geun Lee 4, Ivan O Rosas 5, Jack A Elias 6, Augustine M K Choi 3, Danielle Morse 1
Affiliations
- PMID: 25785991
- PMCID: PMC4364993
- DOI: 10.1371/journal.pone.0121246
Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis
Avignat S Patel et al. PLoS One. 2015.
Abstract
Background: Epithelial cell death is a major contributor to fibrogenesis in the lung. In this study, we sought to determine the function of mitochondria and their clearance (mitophagy) in alveolar epithelial cell death and fibrosis.
Methods: We studied markers of mitochondrial injury and the mitophagy marker, PTEN-induced putative kinase 1 (PINK1), in IPF lung tissues by Western blotting, transmission electron microscopy (TEM), and immunofluorescence. In vitro experiments were carried out in lung epithelial cells stimulated with transforming growth factor-β1 (TGF-β1). Changes in cell function were measured by Western blotting, flow cytometry and immunofluorescence. In vivo experiments were performed using the murine bleomycin model of lung fibrosis.
Results: Evaluation of IPF lung tissue demonstrated increased PINK1 expression by Western blotting and immunofluorescence and increased numbers of damaged mitochondria by TEM. In lung epithelial cells, TGF-β1 induced mitochondrial depolarization, mitochondrial ROS, and PINK1 expression; all were abrogated by mitochondrial ROS scavenging. Finally, Pink1-/- mice were more susceptible than control mice to bleomycin induced lung fibrosis.
Conclusion: TGF-β1 induces lung epithelial cell mitochondrial ROS and depolarization and stabilizes the key mitophagy initiating protein, PINK1. PINK1 ameliorates epithelial cell death and may be necessary to limit fibrogenesis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
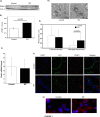
Fig 1. PINK1 Expression and Mitochondrial Dysfunction in IPF Lung Tissue.
A) Western blot of mitochondrial fraction of PINK1 in human lung tissue from control and IPF patients showing increased PINK1 levels in IPF samples. B) Densitometry of blot in A. C) mRNA expression of Pink1 in human lung tissue from control (n = 5) and IPF (n = 5) samples. D) Representative transmission electron micrographs from control and IPF lung tissue showing mitochondria (*). Magnif = 18500x; scale bars = 200nm. E) Quantification of total and abnormal mitochondria. Total mitochondria per μm2 in control and IPF was 0.52 ± 0.39 vs. 0.46 ± 0.34. Abnormal mitochondria per μm2 in control and IPF was 0.12 ± 0.19 vs. 0.32 ± 0.32, p = 0.003. F) Confocal immunofluorescence against PINK1(red) and LC3 (green) in control and IPF lung (magnification 10x). G) Magnified view (60x) of confocal immunofluoresence with DAPI (blue) and PINK1 (red) in control and IPF lung.
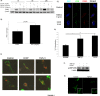
Fig 2. TGF-β1 increases PINK1 expression and induces mitochondrial fission in vitro.
A) TGF-β1 induced PINK1 expression of Beas-2B cells in a time- and dose-dependent manner. B) Quantification by densitometry of PINK1 expression in Beas-2B cells stimulated with TGF-β1 for 6 hours. C) Confocal microscopy of Beas-2B cells stimulated with TGF-β1 (6h) showed that TGF-β1 induces formation of PINK1 puncta (red) (magnification 120x). D) Quantification of colocalization of LC3 and PINK1 punctae (* p = 0.035, ** p = 0.001). E) Confocal microscopy of Beas-2B cells transfected with vectors staining mitochondria (green) and lysosomes (red) and with TGF-β1 stimulation for 6 hours (magnification 60x). F) Western blot of Beas-2B cells stimulated with TGF-β1 (6hrs) showed increased expression of pDRP1 (ser616). G) Confocal microscopy of Beas-2B cells stimulated with TGF-β1 (5 ng/mL, 24 hrs) showed more fragmentation (fission) of mitochondria stained with MitoTracker Green (magnification 120x).
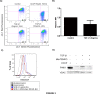
Fig 3. TGF-β1 induces mitochondrial depolarization.
A) Beas-2B cells stimulated with TGF-β1 and CCCP (positive controls) were stained with JC-1 for 15 min and analyzed by flow cytometric analysis. TGF-β1 treated cells showed decreased red fluorescence (mitochondrial depolarization). B) Quantification of relative MFI for experiment in A (p = 0.06). C) Beas-2B cells stimulated with TGF-β1 were stained with MitoSOX for 10 min and analyzed by flow cytometry. TGF-β1 treated cells showed increased red fluorescence (mitochondrial ROS production) and mitochondria-specific antioxidant (MitoTEMPO) reversed the effect of TGF-β1. D) Western blot of the mitochondrial fraction of Beas-2B cells stimulated with TGF-β1 (5ng/mL) +/- MitoTEMPO (200μM) showing decreased PINK1 expression in presence of MitoTEMPO.
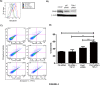
Fig 4. Loss of PINK1 augments TGF-β1 induced cell death.
A) Measurement of mitochondrial ROS in murine type II alveolar epithelial cells from Pink1 -/- and Pink1 WT mice by flow cytometry and MitoSOX staining. B) Western blot against PINK1 demonstrated knockdown of expression with PINK1 siRNA. C) Beas-2B cells were treated with siRNA (50 nM, 24hrs). Then, they were stimulated with TGF-β1 (5 ng/mL, 24hrs) and analyzed by Annexin V/PI flow cytometry. D) Quantification of cell death from experiment in C (*p = 0.0005, **p = 0.0119, ***p = 0.032). Loss of PINK1 exaggerated cell death in cells treated with TGF-β1 relative to transfection with control siRNA.
Fig 5. Loss of PINK1 aggravates bleomycin induced lung fibrosis.
Pink1 -/- mice and their littermate controls (male, 8–10 weeks old) were treated with 3U/kg of intratracheal bleomycin sulfate or saline on day 1 and sacrificed on Day 21. Pink1 -/- mice in the bleomycin group showed higher levels of hydroxyproline compared to controls (85.4 μg/mL vs. 97.0 μg/mL; *p = .05, **p<0.001).
Similar articles
- Mitochondrial 8-oxoguanine DNA glycosylase mitigates alveolar epithelial cell PINK1 deficiency, mitochondrial DNA damage, apoptosis, and lung fibrosis.
Kim SJ, Cheresh P, Jablonski RP, Rachek L, Yeldandi A, Piseaux-Aillon R, Ciesielski MJ, Ridge K, Gottardi C, Lam AP, Pardo A, Selman M, Natarajan V, Kamp DW. Kim SJ, et al. Am J Physiol Lung Cell Mol Physiol. 2020 May 1;318(5):L1084-L1096. doi: 10.1152/ajplung.00069.2019. Epub 2020 Mar 25. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32209025 Free PMC article. - PGAM5 is a key driver of mitochondrial dysfunction in experimental lung fibrosis.
Ganzleben I, He GW, Günther C, Prigge ES, Richter K, Rieker RJ, Mougiakakos D, Neurath MF, Becker C. Ganzleben I, et al. Cell Mol Life Sci. 2019 Dec;76(23):4783-4794. doi: 10.1007/s00018-019-03133-1. Epub 2019 Jun 5. Cell Mol Life Sci. 2019. PMID: 31168659 Free PMC article. - PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis.
Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, Hara H, Minagawa S, Wakui H, Fujii S, Kojima J, Shimizu K, Numata T, Kawaishi M, Odaka M, Morikawa T, Harada T, Nishimura SL, Kaneko Y, Nakayama K, Kuwano K. Ito S, et al. Autophagy. 2015;11(3):547-59. doi: 10.1080/15548627.2015.1017190. Autophagy. 2015. PMID: 25714760 Free PMC article. - PINK1-PARK2-mediated mitophagy in COPD and IPF pathogeneses.
Tsubouchi K, Araya J, Kuwano K. Tsubouchi K, et al. Inflamm Regen. 2018 Oct 24;38:18. doi: 10.1186/s41232-018-0077-6. eCollection 2018. Inflamm Regen. 2018. PMID: 30386443 Free PMC article. Review. - Sphingolipids in pulmonary fibrosis.
Huang LS, Natarajan V. Huang LS, et al. Adv Biol Regul. 2015 Jan;57:55-63. doi: 10.1016/j.jbior.2014.09.008. Epub 2014 Oct 13. Adv Biol Regul. 2015. PMID: 25446881 Free PMC article. Review.
Cited by
- Mechanisms of Bleomycin-induced Lung Fibrosis: A Review of Therapeutic Targets and Approaches.
Mohammed SM, Al-Saedi HFS, Mohammed AQ, Amir AA, Radi UK, Sattar R, Ahmad I, Ramadan MF, Alshahrani MY, Balasim HM, Alawadi A. Mohammed SM, et al. Cell Biochem Biophys. 2024 Sep;82(3):1845-1870. doi: 10.1007/s12013-024-01384-9. Epub 2024 Jul 2. Cell Biochem Biophys. 2024. PMID: 38955925 Review. - The mitophagy pathway and its implications in human diseases.
Wang S, Long H, Hou L, Feng B, Ma Z, Wu Y, Zeng Y, Cai J, Zhang DW, Zhao G. Wang S, et al. Signal Transduct Target Ther. 2023 Aug 16;8(1):304. doi: 10.1038/s41392-023-01503-7. Signal Transduct Target Ther. 2023. PMID: 37582956 Free PMC article. Review. - Candidate genes of idiopathic pulmonary fibrosis: current evidence and research.
Zhou W, Wang Y. Zhou W, et al. Appl Clin Genet. 2016 Feb 2;9:5-13. doi: 10.2147/TACG.S61999. eCollection 2016. Appl Clin Genet. 2016. PMID: 26893575 Free PMC article. Review. - Understanding the molecular regulatory mechanisms of autophagy in lung disease pathogenesis.
Lin L, Lin Y, Han Z, Wang K, Zhou S, Wang Z, Wang S, Chen H. Lin L, et al. Front Immunol. 2024 Oct 31;15:1460023. doi: 10.3389/fimmu.2024.1460023. eCollection 2024. Front Immunol. 2024. PMID: 39544928 Free PMC article. Review. - Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process.
Gustafsson ÅB, Dorn GW 2nd. Gustafsson ÅB, et al. Physiol Rev. 2019 Jan 1;99(1):853-892. doi: 10.1152/physrev.00005.2018. Physiol Rev. 2019. PMID: 30540226 Free PMC article. Review.
References
- Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999; 283: 1488–1493. - PubMed
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004; 304: 1158–1160. - PubMed
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998; 392: 605–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 HL124884/HL/NHLBI NIH HHS/United States
- R01 HL087122/HL/NHLBI NIH HHS/United States
- P01HL114501/HL/NHLBI NIH HHS/United States
- R01HL087122-01/HL/NHLBI NIH HHS/United States
- P01 HL114501/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials