Integration of drug, protein, and gene delivery systems with regenerative medicine - PubMed (original) (raw)
Review
Integration of drug, protein, and gene delivery systems with regenerative medicine
Elizabeth R Lorden et al. Drug Deliv Transl Res. 2015 Apr.
Abstract
Regenerative medicine has the potential to drastically change the field of health care from reactive to preventative and restorative. Exciting advances in stem cell biology and cellular reprogramming have fueled the progress of this field. Biochemical cues in the form of small molecule drugs, growth factors, zinc finger protein transcription factors and nucleases, transcription activator-like effector nucleases, monoclonal antibodies, plasmid DNA, aptamers, or RNA interference agents can play an important role to influence stem cell differentiation and the outcome of tissue regeneration. Many of these biochemical factors are fragile and must act intracellularly at the molecular level. They require an effective delivery system, which can take the form of a scaffold (e.g., hydrogels and electrospun fibers), carrier (viral and nonviral), nano- and microparticle, or genetically modified cell. In this review, we will discuss the history and current technologies of drug, protein, and gene delivery in the context of regenerative medicine. Next, we will present case examples of how delivery technologies are being applied to promote angiogenesis in nonhealing wounds or prevent angiogenesis in age related macular degeneration. Finally, we will conclude with a brief discussion of the regulatory pathway from bench to bedside for the clinical translation of these novel therapeutics.
Conflict of interest statement
Conflict of Interest
Elizabeth Lorden, Howard Levinson, and Kam Leong all declare they have no conflict of interest.
Figures
Fig. 1
Parallel advancements in the use of drugs, proteins, and genes for regenerative therapeutics converged in the 1990’s with the need for rationally designed delivery systems
Fig. 2
Visualization of protein-based cues. Growth factors interact with cells via transmembrane receptors to drive various processes such as differentiation, migration or proliferation (A). Each zinc finger protein recognizes 3–4 base pairs, such that a modular construct of multiple ZFPs can be generated to recognize a unique sequence in the genome (B). Monoclonal antibodies recognize specific antigens within the body, once bound to their target they can either physically inactivate it or recruit immune cells to destroy it (C)
Fig. 3
Delivery vehicles for small molecule drugs, proteins, and nucleic acids. Carriers for soluble factors can be macroscopic, such as hydrogels (A) and electrospun fibers (B), or microscopic, such as micelles and liposomes (C.1), dendrimers (C.2), or particulate hydrogel systems (C.3). Carriers for nucleic acids such as DNA, RNA and plasmid DNA have unique design requirements since they must be able to carry their negatively charged cargo across the negatively charged cellular membrane. Viral carriers (D) can be used to introduce DNA into cells, most commonly done in vitro to generate genetically modified cells (E). Non-viral delivery methods can include simple complexation with cationic polymers (F.1), or the use of particulate systems such as liposomes (F.2) and lipopolyplexes (F.3). These methods can also be used to generate genetically modified cells or deliver nucleic acid cargo in vivo
Fig. 4
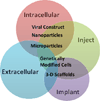
Carrier Selection Guide. Selection of the correct delivery vehicle must first consider whether the soluble factor is active intracellularly or if extracellular delivery is optimal. Once delivery method is chosen, the delivery method of the carrier should be selected based on disease pathogenesis. Injection of microscopic carriers may be optimal for systemic or localized pathogenesis, while implantation of 3D scaffold carriers are most effective when repairing a tissue defect
Fig. 5
Technology Patent Protection and the steps to clinical translation
Similar articles
- Controlled delivery systems: from pharmaceuticals to cells and genes.
Balmayor ER, Azevedo HS, Reis RL. Balmayor ER, et al. Pharm Res. 2011 Jun;28(6):1241-58. doi: 10.1007/s11095-011-0392-y. Epub 2011 Mar 19. Pharm Res. 2011. PMID: 21424163 Review. - Biomaterial-based delivery systems of nucleic acid for regenerative research and regenerative therapy.
Jo JI, Gao JQ, Tabata Y. Jo JI, et al. Regen Ther. 2019 Jul 11;11:123-130. doi: 10.1016/j.reth.2019.06.007. eCollection 2019 Dec. Regen Ther. 2019. PMID: 31338391 Free PMC article. Review. - MicroRNA delivery for regenerative medicine.
Peng B, Chen Y, Leong KW. Peng B, et al. Adv Drug Deliv Rev. 2015 Jul 1;88:108-22. doi: 10.1016/j.addr.2015.05.014. Epub 2015 May 27. Adv Drug Deliv Rev. 2015. PMID: 26024978 Free PMC article. Review. - Nonviral delivery for reprogramming to pluripotency and differentiation.
Park HJ, Shin J, Kim J, Cho SW. Park HJ, et al. Arch Pharm Res. 2014 Jan;37(1):107-19. doi: 10.1007/s12272-013-0287-z. Epub 2013 Nov 13. Arch Pharm Res. 2014. PMID: 24222505 Review. - Entrapment of small molecules and nucleic acid-based drugs in liposomes.
Fenske DB, Cullis PR. Fenske DB, et al. Methods Enzymol. 2005;391:7-40. doi: 10.1016/S0076-6879(05)91001-X. Methods Enzymol. 2005. PMID: 15721372
Cited by
- Genome-editing Technologies for Gene and Cell Therapy.
Maeder ML, Gersbach CA. Maeder ML, et al. Mol Ther. 2016 Mar;24(3):430-46. doi: 10.1038/mt.2016.10. Epub 2016 Jan 12. Mol Ther. 2016. PMID: 26755333 Free PMC article. Review. - Current Concepts of Epigenetics and Its Role in Periodontitis.
Larsson L. Larsson L. Curr Oral Health Rep. 2017;4(4):286-293. doi: 10.1007/s40496-017-0156-9. Epub 2017 Nov 6. Curr Oral Health Rep. 2017. PMID: 29201597 Free PMC article. Review. - Design of Bio-Conjugated Hydrogels for Regenerative Medicine Applications: From Polymer Scaffold to Biomolecule Choice.
Chimisso V, Aleman Garcia MA, Yorulmaz Avsar S, Dinu IA, Palivan CG. Chimisso V, et al. Molecules. 2020 Sep 7;25(18):4090. doi: 10.3390/molecules25184090. Molecules. 2020. PMID: 32906772 Free PMC article. Review. - Protein and mRNA Delivery Enabled by Cholesteryl-Based Biodegradable Lipidoid Nanoparticles.
Li Y, Jarvis R, Zhu K, Glass Z, Ogurlu R, Gao P, Li P, Chen J, Yu Y, Yang Y, Xu Q. Li Y, et al. Angew Chem Int Ed Engl. 2020 Aug 24;59(35):14957-14964. doi: 10.1002/anie.202004994. Epub 2020 Jun 15. Angew Chem Int Ed Engl. 2020. PMID: 32438474 Free PMC article. - Cytosolic delivery of large proteins using nanoparticle-stabilized nanocapsules.
Tang R, Jiang Z, Ray M, Hou S, Rotello VM. Tang R, et al. Nanoscale. 2016 Oct 27;8(42):18038-18041. doi: 10.1039/c6nr07162g. Nanoscale. 2016. PMID: 27738697 Free PMC article.
References
- Hasetine W. A brave new medicine. A conversation with William Haseltine. Interview by Joe Flower. Health Forum journal. 1999;42(4):28–30. 61–65. - PubMed
- Chen FM, Zhao YM, Jin Y, Shi S. Prospects for translational regenerative medicine. Biotechnology advances. 2012;30(3):658–672. - PubMed
- Couto DS, Perez-Breva L, Cooney CL. Regenerative medicine: learning from past examples. Tissue engineering Part A. 2012;18(21–22):2386–2393. - PubMed
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126(4):663–676. doi: http://dx.doi.org/10.1016/j.cell.2006.07.024. - DOI - PubMed
- Davis RLWH, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EB015000/EB/NIBIB NIH HHS/United States
- UH2TR000505/TR/NCATS NIH HHS/United States
- K08 GM085562/GM/NIGMS NIH HHS/United States
- R01 AI096305/AI/NIAID NIH HHS/United States
- R21 EB015300/EB/NIBIB NIH HHS/United States
- UH3 TR000505/TR/NCATS NIH HHS/United States
- UH2 TR000505/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources