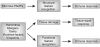Control of adaptive immunity by the innate immune system - PubMed (original) (raw)
Review
Control of adaptive immunity by the innate immune system
Akiko Iwasaki et al. Nat Immunol. 2015 Apr.
Abstract
Microbial infections are recognized by the innate immune system both to elicit immediate defense and to generate long-lasting adaptive immunity. To detect and respond to vastly different groups of pathogens, the innate immune system uses several recognition systems that rely on sensing common structural and functional features associated with different classes of microorganisms. These recognition systems determine microbial location, viability, replication and pathogenicity. Detection of these features by recognition pathways of the innate immune system is translated into different classes of effector responses though specialized populations of dendritic cells. Multiple mechanisms for the induction of immune responses are variations on a common design principle wherein the cells that sense infections produce one set of cytokines to induce lymphocytes to produce another set of cytokines, which in turn activate effector responses. Here we discuss these emerging principles of innate control of adaptive immunity.
Figures
Figure 1
Recognition of structural and functional features. Sensors of the innate immune system recognize infectious agents through detection of unique molecular structures associated with the pathogens (structural feature recognition) or through detection of the characteristic functional activities (functional feature recognition) associated with the infection. The process of infection is often accompanied by tissue damage caused by the pathogens or by the immune response to pathogens. Tissue damage induces a tissue-repair program that can include some specialized immune responses.
Figure 2
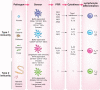
The instruction of various classes of T cell responses by DCs. The development and function of DCs in vivo requires transcription factors that are critical for each stage of their differentiation program. Studies probing the requirement or sufficiency for DC subsets have suggested a relationship between the DC lineage and T cell programming. CTL responses are induced by lymphoid organ–resident, Batf3-dependent CD8α+ DCs and tissue CD103+ DCs (mouse) and the human CD141+ DC counterpart. TH1 cell–mediated immunity requires stimulation, in a GM-CSF-dependent manner, by the CD207+CD103+ DC subset, a minor population of dermal DCs (mouse). TH17 responses are induced by mucosal IRF4-dependent CD103+CD11b+ DCs as well as by skin Langerhans cells, depending on the location of the pathogen (mouse). The human CD1c+ DCs prime TH17 cell responses. Finally, TH2 cell–mediated immunity requires IRF4-dependent CD301b+CD11b+ DCs (mouse). These DCs comprise the majority of dermal DC (dDC) population and are distinct from CD207+ CD103+ dermal DCs. In contrast, human Langerhans cells induce TH2 cytokine secretion ex vivo.
Figure 3
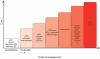
The order of effector function engagement, from lowest cost to highest cost. Defense mechanisms of the immune system have different costs to host fitness. The order of engagement of effector functions corresponds to their increasing costs (here, their potential to cause immunopathology). The low-cost effector mechanisms, such as antimicrobial peptides (AMPs), secretory IgA and circulatory IgM, are expressed constitutively. When they are insufficient to contain pathogens, the response of next lowest cost is induced (here, tissue-resident macrophages); when this response is insufficient, neutrophils are recruited, and so on. The examples of effector responses presented here and their costs are for illustration purposes only; they are neither comprehensive nor quantitative.
Figure 4
The two-tiered design of the immune responses. Immune responses are orchestrated by three categories of cells that function as ‘sensors’ that detect pathogens and secrete ‘level 1’ cytokines, the tissue-resident lymphocytes that respond to ‘level 1’ cytokines to secrete ‘level 2’ cytokines, and the effector cells that respond to ‘level 2’ cytokines to carry out effector functions to eliminate the pathogen. DCs and macrophages serve as sensors for the type 1 immune response and epithelial cells and mast cells for the type 2 immune response. ‘Level 1’ cytokines act on terminally differentiated lymphocytes, including ILCs, ILLs, TFH cells and TRM cells. In response to ‘level 1’ cytokines, these lymphocytes produce ‘level 2’ cytokines. The ‘level 2’ cytokines act on the effector cells of the immune response. These include macrophages, neutrophils, epithelial cells, eosinophils and basophils, B cells as well as sensory neurons, endothelium and smooth muscle cells. These cells perform diverse effector functions, including barrier defenses, killing and expulsion of pathogens, antibody production and tissue repair.
Figure 5
Single-tiered immune responses. Some immune responses involve only the ‘level 1’ signals that act directly on the effector cells. For antiviral responses, plasmacytoid DCs serve as a key sensor for the detection of viruses through endosomal TLRs to induce type 1 interferons, which block viral replication. Mast cells that reside in the mucosa sense the presence of the noxious substances and parasitic worms and release histamines and prostaglandins, which act on local vasculature to induce vasodilation and leakage, and intestinal and airway smooth muscles to induce peristalsis, contraction and expulsion of parasites. Histamine produced by mast cells and TSLP produced by epithelial cells can also stimulate a subset of C-fiber neurons to induce the itching sensation.
Similar articles
- Adaptive immunity to fungi.
Wüthrich M, Deepe GS Jr, Klein B. Wüthrich M, et al. Annu Rev Immunol. 2012;30:115-48. doi: 10.1146/annurev-immunol-020711-074958. Epub 2012 Jan 3. Annu Rev Immunol. 2012. PMID: 22224780 Free PMC article. Review. - Innate immune responses to respiratory syncytial virus infection.
Mukherjee S, Lukacs NW. Mukherjee S, et al. Curr Top Microbiol Immunol. 2013;372:139-54. doi: 10.1007/978-3-642-38919-1_7. Curr Top Microbiol Immunol. 2013. PMID: 24362688 Review. - Immunity to respiratory viruses.
Kohlmeier JE, Woodland DL. Kohlmeier JE, et al. Annu Rev Immunol. 2009;27:61-82. doi: 10.1146/annurev.immunol.021908.132625. Annu Rev Immunol. 2009. PMID: 18954284 Review. - Cytokines in innate and adaptive immunity.
Banyer JL, Hamilton NH, Ramshaw IA, Ramsay AJ. Banyer JL, et al. Rev Immunogenet. 2000;2(3):359-73. Rev Immunogenet. 2000. PMID: 11256745 Review. - Receptors and pathways in innate antifungal immunity: the implication for tolerance and immunity to fungi.
Zelante T, Montagnoli C, Bozza S, Gaziano R, Bellocchio S, Bonifazi P, Moretti S, Fallarino F, Puccetti P, Romani L. Zelante T, et al. Adv Exp Med Biol. 2007;590:209-21. doi: 10.1007/978-0-387-34814-8_15. Adv Exp Med Biol. 2007. PMID: 17191388 Review. No abstract available.
Cited by
- NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer.
Faria SS, Costantini S, de Lima VCC, de Andrade VP, Rialland M, Cedric R, Budillon A, Magalhães KG. Faria SS, et al. J Biomed Sci. 2021 Apr 12;28(1):26. doi: 10.1186/s12929-021-00724-8. J Biomed Sci. 2021. PMID: 33840390 Free PMC article. Review. - Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases.
Alanazi HH, Elasbali AM, Alanazi MK, El Azab EF. Alanazi HH, et al. Molecules. 2023 Dec 12;28(24):8045. doi: 10.3390/molecules28248045. Molecules. 2023. PMID: 38138535 Free PMC article. Review. - Yersinia enterocolitica YopH-Deficient Strain Activates Neutrophil Recruitment to Peyer's Patches and Promotes Clearance of the Virulent Strain.
Dave MN, Silva JE, Eliçabe RJ, Jeréz MB, Filippa VP, Gorlino CV, Autenrieth S, Autenrieth IB, Di Genaro MS. Dave MN, et al. Infect Immun. 2016 Oct 17;84(11):3172-3181. doi: 10.1128/IAI.00568-16. Print 2016 Nov. Infect Immun. 2016. PMID: 27550935 Free PMC article. - Editorial: Updates on toll-like receptors in cancer immunity and immunotherapy.
Hoden B, Nagai Y, Schuettpelz L, Zhang D. Hoden B, et al. Front Immunol. 2023 Nov 13;14:1331317. doi: 10.3389/fimmu.2023.1331317. eCollection 2023. Front Immunol. 2023. PMID: 38022608 Free PMC article. No abstract available. - Gasdermins in sepsis.
Wang W, He Z. Wang W, et al. Front Immunol. 2023 Nov 3;14:1203687. doi: 10.3389/fimmu.2023.1203687. eCollection 2023. Front Immunol. 2023. PMID: 38022612 Free PMC article. Review.
References
- Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54:1–13. - PubMed
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. - PubMed
- Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 2014;32:461–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI089771/AI/NIAID NIH HHS/United States
- AI089771/AI/NIAID NIH HHS/United States
- AI064705/AI/NIAID NIH HHS/United States
- R01 AI064705/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI054359/AI/NIAID NIH HHS/United States
- AI046688/AI/NIAID NIH HHS/United States
- R37 AI046688/AI/NIAID NIH HHS/United States
- R56 AI062428/AI/NIAID NIH HHS/United States
- R01 DK071754/DK/NIDDK NIH HHS/United States
- R01 AI062428/AI/NIAID NIH HHS/United States
- AI081884/AI/NIAID NIH HHS/United States
- R01 AI081884/AI/NIAID NIH HHS/United States
- DK071754/DK/NIDDK NIH HHS/United States
- R01 AI046688/AI/NIAID NIH HHS/United States
- AI062428/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources