Nicotinic Mechanisms Modulate Ethanol Withdrawal and Modify Time Course and Symptoms Severity of Simultaneous Withdrawal from Alcohol and Nicotine - PubMed (original) (raw)
Nicotinic Mechanisms Modulate Ethanol Withdrawal and Modify Time Course and Symptoms Severity of Simultaneous Withdrawal from Alcohol and Nicotine
Erika Perez et al. Neuropsychopharmacology. 2015 Sep.
Abstract
Alcohol and nicotine are among the top causes of preventable death in the United States. Unfortunately, people who are dependent on alcohol are more likely to smoke than individuals in the general population. Similarly, smokers are more likely to abuse alcohol. Alcohol and nicotine codependence affects health in many ways and leads to poorer treatment outcomes in subjects who want to quit. This study examined the interaction of alcohol and nicotine during withdrawal and compared abstinence symptoms during withdrawal from one of the two drugs only vs both. Our results indicate that simultaneous withdrawal from alcohol and nicotine produces physical symptoms that are more severe and last longer than those experienced during withdrawal from one of the two drugs alone. In animals experiencing withdrawal after chronic ethanol treatment, acute nicotine exposure was sufficient to prevent abstinence symptoms. Similarly, symptoms were prevented when alcohol was injected acutely in mice undergoing nicotine withdrawal. These experiments provide evidence for the involvement of the nicotinic cholinergic system in alcohol withdrawal. Furthermore, the outcomes of intracranial microinfusions of mecamylamine, a nonselective nicotinic receptor antagonist, highlight a major role for the nicotinic receptors expressed in medial habenula and interpeduncular nucleus during withdrawal. Overall, the data support the notion that modulating the nicotinic cholinergic system might help to maintain long-term abstinence from alcohol.
Figures
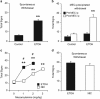
Figure 1
nAChRs modulate physical signs of ethanol withdrawal. (a, b) Control- and ethanol (ETOH)-treated mice were tested for changes in physical signs during spontaneous (a) or mecamylamine-precipitated withdrawal (MEC, b). Only ethanol-treated mice displayed significant increases in physical signs. (c, d) Mice were treated with either ethanol injections or nicotine (NIC) in their drinking water. Physical signs were measured following various doses of the nicotinic receptor antagonist, mecamylamine (c) or during spontaneous withdrawal (d). Although ethanol-treated mice were more sensitive to the effects of mecamylamine (c), during spontaneous withdrawal they exhibited symptoms similar to those observed in nicotine-withdrawing mice (d). Animal numbers are as follows: (a) 8 per experimental group; (b) 5 Control, 7 ETOH; (c, d) 6 per experimental group. **_P_>0.01 compared with control and Mec (0) group, #_p_>0.01 compared with NIC at same dose.
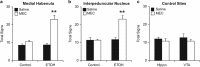
Figure 2
MHb and IPN, but not hippocampus or VTA, play a role in modulating physical signs of ethanol withdrawal. (a, b) Mice treated with either control or ethanol (ETOH) injections received saline or mecamylamine (MEC) infusions into either MHB or IPN. Physical signs increased significantly after mecamylamine infusions only in mice treated with ethanol. (c) Hippocampus (Hippo)- and VTA-cannulated mice were treated with ethanol and received infusions of saline or mecamylamine. Physical signs did not increase after mecamylamine treatment in these experimental subjects. Animal numbers are as follows: (a) 5 Controls and 9 ETOH; (b) 8 Controls and 13 ETOH; (c) 6 Hippo and 5 VTA. **P<0.01 compared with all groups.
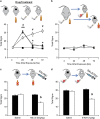
Figure 3
In codependent mice, simultaneous withdrawal produces long-lasting symptoms that are attenuated by continued exposure to either nicotine or ethanol. (a) Mice were treated with either control, ethanol (ETOH), nicotine (NIC), or both. Physical signs were observed 4, 24, 48, and 72 h after drug cessation. All drug groups exhibited increases in signs at 24 h, but only simultaneous withdrawal of ethanol and nicotine produced sustained symptoms that persisted at least for 72 h after treatment. (b). Cotreated mice underwent ethanol-only or nicotine-only withdrawal. Continued exposure to at least one drug protected against the emergence of somatic signs during withdrawal. (c, d) Mice treated with either chronic ethanol only (c) or chronic nicotine only (d) were observed for changes in physical signs during spontaneous withdrawal. Mice were then treated with an acute administration of either saline, 0.3 mg/kg nicotine (c), or 1 g/kg ethanol (d), and physical signs were reevaluated. Before acute injections, mice undergoing withdrawal exhibited significant physical signs that were decreased after either alcohol or nicotine treatment. Animal numbers are as follows: (a) 15 no drug, 10 NIC, 11 ETOH, and 8 cotreated; (b) 7 NIC and 8 ETOH; (c) 10 Saline and 9 NIC; (d) 10 Saline and 7 ETOH. *P<0.05, **P<0.01 compared to control; #p<0.01 cotreated compared with control and single treatment; &p<0.01 compared with preinjection.
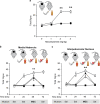
Figure 4
nAChRs within the MHb and the IPN influence withdrawal symptoms in mice cotreated with nicotine and ethanol. All mice were either injected with saline and drank water with saccharin or received ethanol (ETOH) injections and nicotine (NIC) in the drinking water. (a) Mice received i.p. injections of mecamylamine at various doses to precipitate withdrawal. Physical signs increased significantly only after 2 and 3 mg/kg mecamylamine. (b, c) Mice underwent the following withdrawal scenarios: ethanol only, nicotine only, or simultaneous withdrawal from both nicotine and ethanol. Mice received saline infusions into MHb (b) or IPN (c) at 4, 24, and 72 h after drug cessation. A physical withdrawal syndrome was precipitated with mecamylamine (1 μg) at 48 h. The nAChR antagonist triggered withdrawal symptoms in the asymptomatic animals allowed access to one drug during withdrawal from the other. Mecamylamine had no effect on control mice and did not exacerbate withdrawal symptoms in mice undergoing withdrawal from both nicotine and ethanol. Animal numbers are as follows: (a) 6 per experimental group; (b) 8 Control, 5 NIC, 5 ETOH, and 5 both; (c) 8 Control, 6 NIC, 7 ETOH, and 6 both. **P<0.01 compared with no-drug control.
Similar articles
- Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice.
Salas R, Sturm R, Boulter J, De Biasi M. Salas R, et al. J Neurosci. 2009 Mar 11;29(10):3014-8. doi: 10.1523/JNEUROSCI.4934-08.2009. J Neurosci. 2009. PMID: 19279237 Free PMC article. - Sex differences in cholinergic systems in the interpeduncular nucleus following nicotine exposure and withdrawal.
Correa VL, Flores RJ, Carcoba LM, Arreguin MC, O'Dell LE. Correa VL, et al. Neuropharmacology. 2019 Nov 1;158:107714. doi: 10.1016/j.neuropharm.2019.107714. Epub 2019 Jul 17. Neuropharmacology. 2019. PMID: 31325431 Free PMC article. - Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms.
Zhao-Shea R, Liu L, Pang X, Gardner PD, Tapper AR. Zhao-Shea R, et al. Curr Biol. 2013 Dec 2;23(23):2327-35. doi: 10.1016/j.cub.2013.09.041. Epub 2013 Nov 14. Curr Biol. 2013. PMID: 24239118 Free PMC article. - The habenulo-interpeduncular pathway in nicotine aversion and withdrawal.
Antolin-Fontes B, Ables JL, Görlich A, Ibañez-Tallon I. Antolin-Fontes B, et al. Neuropharmacology. 2015 Sep;96(Pt B):213-22. doi: 10.1016/j.neuropharm.2014.11.019. Epub 2014 Dec 2. Neuropharmacology. 2015. PMID: 25476971 Free PMC article. Review. - The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation.
McLaughlin I, Dani JA, De Biasi M. McLaughlin I, et al. J Neurochem. 2017 Aug;142 Suppl 2(Suppl 2):130-143. doi: 10.1111/jnc.14008. J Neurochem. 2017. PMID: 28791703 Free PMC article. Review.
Cited by
- Interventions for pregnant women who use tobacco and other substances: a systematic review protocol.
Jackson MA, Baker AL, McCarter KL, Brown AL, Gould GS, Dunlop AJ. Jackson MA, et al. BMJ Open. 2019 Nov 11;9(11):e032449. doi: 10.1136/bmjopen-2019-032449. BMJ Open. 2019. PMID: 31719091 Free PMC article. - Mutation of the α5 nicotinic acetylcholine receptor subunit increases ethanol and nicotine consumption in adolescence and impacts adult drug consumption.
Quijano Cardé NA, Shaw J, Carter C, Kim S, Stitzel JA, Venkatesh SK, Ramchandani VA, De Biasi M. Quijano Cardé NA, et al. Neuropharmacology. 2022 Sep 15;216:109170. doi: 10.1016/j.neuropharm.2022.109170. Epub 2022 Jun 22. Neuropharmacology. 2022. PMID: 35752273 Free PMC article. - Exposure to fruit-flavoring during adolescence increases nicotine consumption and promotes dose escalation.
Patten T, Dreier A, Herman RJ, Kimball BA, De Biasi M. Patten T, et al. Neuropharmacology. 2021 Sep 1;195:108672. doi: 10.1016/j.neuropharm.2021.108672. Epub 2021 Jun 19. Neuropharmacology. 2021. PMID: 34153314 Free PMC article. - How Imaging Glutamate, γ-Aminobutyric Acid, and Dopamine Can Inform the Clinical Treatment of Alcohol Dependence and Withdrawal.
Hillmer AT, Mason GF, Fucito LM, O'Malley SS, Cosgrove KP. Hillmer AT, et al. Alcohol Clin Exp Res. 2015 Dec;39(12):2268-82. doi: 10.1111/acer.12893. Epub 2015 Oct 28. Alcohol Clin Exp Res. 2015. PMID: 26510169 Free PMC article. Review. - Effect of Combination Treatment With Varenicline and Nicotine Patch on Smoking Cessation Among Smokers Who Drink Heavily: A Randomized Clinical Trial.
King A, Vena A, de Wit H, Grant JE, Cao D. King A, et al. JAMA Netw Open. 2022 Mar 1;5(3):e220951. doi: 10.1001/jamanetworkopen.2022.0951. JAMA Netw Open. 2022. PMID: 35244704 Free PMC article. Clinical Trial.
References
- Asher MK, Martin RA, Rohsenow DJ, MacKinnon SV, Traficante R, Monti PM. Perceived barriers to quitting smoking among alcohol dependent patients in treatment. J Subst Abuse Treat. 2003;24:169–174. - PubMed
- Berrettini WH, Doyle GA. The CHRNA5-A3-B4 gene cluster in nicotine addiction. Molecular psychiatry. 2012;17:856–866. - PubMed
- Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92:15–26. - PubMed
- CASA 2000CASA's cost of living adjustment, using the Bureau of Labor Statistics Inflation Calculator, of 1998 data by Harwood, H. Report prepared by The Lewin Group for the National Institute on Alcohol Abuse and Alcoholism, 2000. Based on estimates, analyses, and datareported in Harwood, H., Fountain, D., and Livermore, G. (1992). The Economic Costs of Alcohol and Drug Abuse in the United States. Report prepared for the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism National Institutes of Health, U.S. Department of Health and Human Services. NIH Publication No 98-4327. (2005). Rockville, MD.
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008076/GM/NIGMS NIH HHS/United States
- DA017173/DA/NIDA NIH HHS/United States
- R01 DA017173/DA/NIDA NIH HHS/United States
- R21 DA024385/DA/NIDA NIH HHS/United States
- DA024385/DA/NIDA NIH HHS/United States
- F31 AA018626/AA/NIAAA NIH HHS/United States
- F31AA018626/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources