Pharmacokinetics (PK), pharmacodynamics (PD) and integrated PK/PD modeling of a novel long acting FGF21 clinical candidate PF-05231023 in diet-induced obese and leptin-deficient obese mice - PubMed (original) (raw)
Pharmacokinetics (PK), pharmacodynamics (PD) and integrated PK/PD modeling of a novel long acting FGF21 clinical candidate PF-05231023 in diet-induced obese and leptin-deficient obese mice
Yan Weng et al. PLoS One. 2015.
Abstract
Pharmacological administration of fibroblast growth factor 21 (FGF21) improves metabolic profile in preclinical species and humans. FGF21 exerts its metabolic effects through formation of beta-klotho (KLB)/FGF receptor 1c FGFR1c complex and subsequent signaling. Data from various in vitro systems demonstrate the intact C- and N-terminus of FGF21 is required for binding with KLB, and interaction with FGFR1c, respectively. However the relative roles of the termini for in vivo pharmacological effects are unclear. Here we report PF-05231023, a long-acting FGF21 analogue which is unique in that the half-life and subcutaneous (s.c.) bioavailability of the intact C-terminus are significantly different from those of the intact N-terminus (2 vs. 22 hr for half-life and 4~7 vs. ~50% SC bioavailability). Therefore, this molecule serves as a valuable tool to evaluate the relative roles of intact C-terminus vs. N-terminus in in vivo pharmacology studies in preclinical species. We determined the effects of PF-05231023 administration on body weight (BW) loss and glucose reduction during an oral glucose tolerance test (OGTT) following SC and intravenous (i.v.) administration in diet-induced obese (DIO) and leptin-deficient obese (ob/ob) mice, respectively. Our data show that the intact N-terminus of FGF21 in PF-05231023 appears to be sufficient to drive glucose lowering during OGTT and sustain BW loss in DIOs. Further, PK/PD modeling suggests that while the intact FGF21 C-terminus is not strictly required for glucose lowering during OGTT in ob/ob mice or for BW reduction in DIO mice, the higher potency conferred by intact C-terminus contributes to a rapid initiation of pharmacodynamic effects immediately following dosing. These results provide additional insight into the strategy of developing stabilized versions of FGF21 analogs to harness the full spectrum of its metabolic benefits.
Conflict of interest statement
Competing Interests: All authors were employees of Pfizer during the completion and analyses of these studies. Pfizer has provided all funding for these studies. This does not alter the authors’ adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures
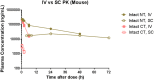
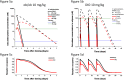
Fig 1. Plasma concentration-time curves of PF-05231023 intact CT and NT.
Plasma concentration-time curves of PF-05231023 in DIO mice following a single IV and SC administration at 10 mg/kg. 3 animals were used per time point. n = 3 animals were used for the 10 mg/kg IV and n = 4 animals were used for the rest of the groups.
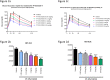
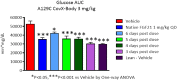
Fig 2. Glucose lowering during OGTT in ob/ob mice upon IV and SC administration of PF-05231023.
Male ob/ob mice approximately 9 weeks of age were administered PF-05231023 IV (a) or SC (c) and OGTT was performed 72 hours later, after an overnight fast. At least 8 mice were used per dose group for the OGTTs. Area under the curve during OGTT after IV (b) or SC (d) administration of PF-05231023. 8 animals were used per group. * p < 0.05, ** p < 0.01, and *** p < 0.001 using one-way Anova with Dunnett’s post hoc test.
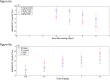
Fig 3. Glucose lowering during OGTT after PF-05231023 administration.
Male ob/ob mice approximately 9 weeks of age were administered 3 mg/kg PF-05231023 SC and OGTT was conducted after an overnight fast on the indicated days. At least 8 animals were used per group per day. * p < 0.05, ** p < 0.01, and *** p < 0.001 using one-way Anova with Dunnett’s post hoc test.
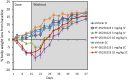
Fig 4. Percentage BW loss in DIO mice following IV and SC administration of PF-05231023.
Male DIO mice at approximately 14 weeks on 60% HFD were administered PF-05231023 IV or SC at the indicated doses on Day 1, 8 and 15. Body weight was measured twice a week throughout the course of the study until day 57. At least 9 animals were used per dose group.
Fig 5. PK and RO time courses for modeled scenarios.
(a,b) Calculated intact (solid line) and N-only (dashed line) following IV (black) or SC (red) administration of 10 mg/kg of PF-05231023. The in vitro potency of the intact molecule is shown in blue, and the estimated in vivo potency of the N-only pool is shown in green. As the model includes linear PK, other doses can be considered by simple shifting of the curves up or down by the relative dose. The results for central compartment levels in the ob/ob mouse model are in (a); the curves for adipose exposure in DIO mice are in (b). (c,d) Calculated receptor occupancy in the central space of ob/ob mice (c) or the adipose space of DIO mice (d) for the 10 mg/kg dose delivered IV (black) or SC (red). The rapid but short-lasting rise in RO following dosing driven by intact molecules is evident, followed by a sustained RO by the N-only molecules.
Fig 6. OGTT modeling in ob/ob mice.
(a) Data and model predictions for OGTT performed 3, 4, 5, and 6 days following single 3 (red) or 10 (blue) mg/kg SC doses of PF-05231023. (b) Day 3 dose response to single 0.03–10 mg/kg PF-05231023 delivered IV (red) or SC (blue) on day 0. Data represented by circles and error bars; model predictions represented by x’s.
Fig 7. BW loss model results in DIO mice.
(a) Optimized model parameter values, as described in the text. (b,c) Data (shapes) and model predictions (curves) for BW changes following three once-weekly doses of PF-05231023 delivered IV (b) or SC (c) starting on day 0. (d) Optimized transfer function (function of ρ and δ; P(RO) in the text) relating the effect on BW changes to receptor occupancy.
Similar articles
- Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study.
Dong JQ, Rossulek M, Somayaji VR, Baltrukonis D, Liang Y, Hudson K, Hernandez-Illas M, Calle RA. Dong JQ, et al. Br J Clin Pharmacol. 2015 Nov;80(5):1051-63. doi: 10.1111/bcp.12676. Epub 2015 Jul 29. Br J Clin Pharmacol. 2015. PMID: 25940675 Free PMC article. Clinical Trial. - Mechanistic investigation of the preclinical pharmacokinetics and interspecies scaling of PF-05231023, a fibroblast growth factor 21-antibody protein conjugate.
Giragossian C, Vage C, Li J, Pelletier K, Piché-Nicholas N, Rajadhyaksha M, Liras J, Logan A, Calle RA, Weng Y. Giragossian C, et al. Drug Metab Dispos. 2015 Jun;43(6):803-11. doi: 10.1124/dmd.114.061713. Epub 2015 Mar 24. Drug Metab Dispos. 2015. PMID: 25805881 - PF-05231023, a long-acting FGF21 analogue, decreases body weight by reduction of food intake in non-human primates.
Thompson WC, Zhou Y, Talukdar S, Musante CJ. Thompson WC, et al. J Pharmacokinet Pharmacodyn. 2016 Aug;43(4):411-25. doi: 10.1007/s10928-016-9481-1. Epub 2016 Jul 12. J Pharmacokinet Pharmacodyn. 2016. PMID: 27405817 Free PMC article. - Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21.
Iglesias P, Selgas R, Romero S, Díez JJ. Iglesias P, et al. Eur J Endocrinol. 2012 Sep;167(3):301-9. doi: 10.1530/EJE-12-0357. Epub 2012 Jun 27. Eur J Endocrinol. 2012. PMID: 22740503 Review. - FGF21-receptor agonists: an emerging therapeutic class for obesity-related diseases.
Sonoda J, Chen MZ, Baruch A. Sonoda J, et al. Horm Mol Biol Clin Investig. 2017 May 19;30(2):/j/hmbci.2017.30.issue-2/hmbci-2017-0002/hmbci-2017-0002.xml. doi: 10.1515/hmbci-2017-0002. Horm Mol Biol Clin Investig. 2017. PMID: 28525362 Review.
Cited by
- Use of FGF21 analogs for the treatment of metabolic disorders: a systematic review and meta-analysis.
Carbonetti MP, Almeida-Oliveira F, Majerowicz D. Carbonetti MP, et al. Arch Endocrinol Metab. 2023 Nov 10;68:e220493. doi: 10.20945/2359-4292-2022-0493. Arch Endocrinol Metab. 2023. PMID: 37948566 Free PMC article. Review. - The effect of hydration status on plasma FGF21 concentrations in humans: A subanalysis of a randomised crossover trial.
Carroll HA, Chen YC, Templeman I, James LJ, Betts JA, Trim WV. Carroll HA, et al. PLoS One. 2020 Aug 5;15(8):e0235557. doi: 10.1371/journal.pone.0235557. eCollection 2020. PLoS One. 2020. PMID: 32756564 Free PMC article. Clinical Trial. - Fibroblast Growth Factor 21 and the Adaptive Response to Nutritional Challenges.
Martínez-Garza Ú, Torres-Oteros D, Yarritu-Gallego A, Marrero PF, Haro D, Relat J. Martínez-Garza Ú, et al. Int J Mol Sci. 2019 Sep 21;20(19):4692. doi: 10.3390/ijms20194692. Int J Mol Sci. 2019. PMID: 31546675 Free PMC article. Review. - Targeting adipose tissue in the treatment of obesity-associated diabetes.
Kusminski CM, Bickel PE, Scherer PE. Kusminski CM, et al. Nat Rev Drug Discov. 2016 Sep;15(9):639-660. doi: 10.1038/nrd.2016.75. Epub 2016 Jun 3. Nat Rev Drug Discov. 2016. PMID: 27256476 Review. - FGF21 Regulates Sweet and Alcohol Preference.
Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, Bernardo B, Müller CP, Tang H, Mangelsdorf DJ, Goodwin B, Kliewer SA. Talukdar S, et al. Cell Metab. 2016 Feb 9;23(2):344-9. doi: 10.1016/j.cmet.2015.12.008. Epub 2015 Dec 24. Cell Metab. 2016. PMID: 26724861 Free PMC article.
References
- Gimeno RE, Moller DE FGF21-based pharmacotherapy—potential utility for metabolic disorders. Trends Endocrinol Metab. 2014; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
Pfizer Inc. provided funding for this study. The funder provided support in the form of salaries for all authors listed, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous