Cotranslational stabilization of Sec62/63 within the ER Sec61 translocon is controlled by distinct substrate-driven translocation events - PubMed (original) (raw)
Cotranslational stabilization of Sec62/63 within the ER Sec61 translocon is controlled by distinct substrate-driven translocation events
Brian J Conti et al. Mol Cell. 2015.
Abstract
The ER Sec61 translocon is a large macromolecular machine responsible for partitioning secretory and membrane polypeptides into the lumen, cytosol, and lipid bilayer. Because the Sec61 protein-conducting channel has been isolated in multiple membrane-derived complexes, we determined how the nascent polypeptide modulates translocon component associations during defined cotranslational translocation events. The model substrate preprolactin (pPL) was isolated principally with Sec61αβγ upon membrane targeting, whereas higher-order complexes containing OST, TRAP, and TRAM were stabilized following substrate translocation. Blocking pPL translocation by passenger domain folding favored stabilization of an alternate complex that contained Sec61, Sec62, and Sec63. Moreover, Sec62/63 stabilization within the translocon occurred for native endogenous substrates, such as the prion protein, and correlated with a delay in translocation initiation. These data show that cotranslational translocon contacts are ultimately controlled by the engaged nascent chain and the resultant substrate-driven translocation events.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
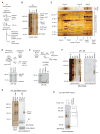
Figure 1. Nascent pPL-86 Binds to a Subset of ER-Derived RAMPs
(A) Schematic of protocol used to isolate ribosome-associated membrane proteins (RAMPs). (B) Blue native (BN) - PAGE analysis of RAMPs labeled with translocon complexes. (C) 2nd dimension SDS-PAGE analysis of a BN-PAGE gel slice (silver stain) containing translocon components and RAMP complexes as labeled. Asterisks denote TRAP components in the 750 kD RAMP complex. (D) pPL-86 ribosome-translocon complexes (RTCs) were solubilized and pelleted (pel) by ultracentrifugation and analyzed by SDS-PAGE (autoradiogram). Cleared lysate (lys, cl) shows little pPL-86 remaining in the supernatant (sup). (E) SDS-PAGE (autoradiogram) of pPL-86 RTCs before and after NaCl and puromycin (puro) treatment followed by fractionation using ultracentrifugation into RAMP fractions (arrow) and ribosome pellets (rib). (F) BN-PAGE of cleared lysate, wash (W), and RAMP fractions obtained from pPL-86 in vitro translation reactions. (G) pPL-86 ribosome-nascent chain complexes with or without addition of solubilized membranes (RNCs or RNCs + Mb) did not associate with RAMP complexes on BN-PAGE. (H) Incubation of pPL-86 RAMP complexes at 24°C prior to BN-PAGE. Percent nascent chain remaining associated with each RAMP complex after incubation is shown (± SEM, n=3). See also Figure S1.
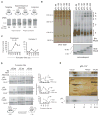
Figure 2. Nascent Chain Translocation Stabilizes Higher-Order RAMP Complexes
(A) SDS-PAGE of translations ± CRMs at indicated pPL truncations. tRNA was removed with RNase. (B) BN- and SDS-PAGE of RAMPs, corresponding to translations (+CRMs) in panel A. Major complexes A–E are indicated. (C) Mean percent of RAMPs recovered in complexes A and B or C at specified chain lengths ± SEM (n=6). (D) SDS-PAGE of truncated single-cysteine pPL mutants (Cys34, Cys56, Cys88) expressed in CRMs and treated with 0.5M NaCl or digitonin (dig) prior to pegylation (PEG-mal). “*” and “←” indicate unmodified and pegylated peptidyl-tRNA respectively. Schematic diagram illustrates effects of salt and digitonin on assembled RTC intermediates. Mean percent pegylation is shown ± SEM (n ≥ 3). (E) BN-PAGE of pPL-137 RAMPs (top) aligned with corresponding 2D-BN/SDS-PAGE (bottom) labeled with complexes A–E. Silver stains, color; autoradiograms, grayscale. See also Figure S2.
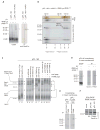
Figure 3. Nascent pPL-Associated RAMPs Contain Sec61, OST, TRAM, and TRAP
(A) Translation of pPL-163-114UAG in presence of ANB-Lys-tRNAUAG incorporated ANB-Lys into position 114. UV light exposure as indicated (left) induced a 55 kD adduct (arrows) identified as Sec61α by immunoprecipitation (IP) (right). Peptidyl-tRNA was removed with RNase. SDS-PAGE autoradiograms are shown. (B) 2D-BN/SDS-PAGE analysis of photocrosslinked RAMPs showing location of Sec61α photoadduct (autoradiogram) and migration of complexes A–E. (C) Antibody (Ig) and Fab gel-shifts ± indicated peptide as performed on pPL-163-derived RAMPs and analyzed by BN-PAGE (autoradiogram). Arrows indicate changes in band A-E migration. Asterisks denote new autoradiographic signal intensity on top of band C or D resulting from gel-shifts of lower MW bands. Gel-shift subpanels were cropped from separate experiments performed identically. (D) ANB-Lys incorporation into pPL-86 lysine residues by translation in the presence of ANB-Lys-tRNALys produced a 45 kD photo-adduct (arrow) with TRAM as identified by immunoprecipitation (IP). SDS-PAGE autoradiograms are shown. (E) Preventing signal cleavage by pPL mutation (pPL-mutSS as in Figure S2) retained TRAM photocrosslinking (arrow) at truncation 163 as performed in panel D and as revealed by IP of UV-exposed lysates. SDS-PAGE autoradiograms are shown. (F) Western blots (IB) on SDS-PAGE of indicated RAMP fractions for TRAM and Sec61α simultaneously. IPs and IBs within panels were cropped from non-adjacent lanes on the same gel..
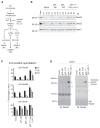
Figure 4. Blocking pPL Translocation Stabilizes RAMP Complex C
(A) Schematic of pPL45-Zn construct showing effect of Zn+2 on passenger translocation. (B) SDS-PAGE of pPL and pPL45-Zn translated ± CRMS, ± Zn+2 both before and after proteinase K digestion (PK). (C) BN- and SDS-PAGE of RAMPs derived from the indicated pPL45-Zn truncations that were produced in the presence (right) and absence (left) of Zn+2. (D) Mean percent of RAMPs recovered in complex C at specified chain lengths ± SEM (n=3). (E) From top to bottom. Silver stain and corresponding autoradiogram of 1D BN-PAGE gel from pPL55-Zn construct truncated at indicated residues and translated in presence of Zn+2. Middle panel is a silver-stained representative 2D-BN/SDS-PAGE gel aligned with upper gel slices showing composition of RAMP complexes. Bottom panels show autoradiograms of 2D-BN/SDS-PAGE gels derived from indicated translation reactions. (F),(G) Percent of nascent chains remaining associated with each RAMP complex after 24°C incubation for pPL-163 and pPL45-Zn-163 (produced in the presence of Zn+2) ± SEM, n=3. See also Figure S3.
Figure 5. RAMP Complex C Contains Sec61, Sec62, and Sec63
(A) Purification scheme used to isolate and identify pPL-bound RAMP components. “-His” refers to truncations encoding a 10x His-tag. In panels (B)–(E), all RAMPs were derived from translations in the presence of Zn+2. (B) BN-PAGE of pPL45-Zn-163-His RAMPs treated with DMSO or DTSSP. (C) BN-PAGE (silver stain) of pPL45-Zn-163-His RAMP purifications confirmed complex C isolation. (D) SDS-PAGE of purified pPL45-Zn-163-His RAMPs, compared to those of mock control and pPL-163-His, indicated isolation of 55 and 100 kD proteins (top, silver stain, “←*”). Western blot (bottom) identified the 38 kD band as Sec61α. (E) Samples from experiment 1 and 2, as prepared and designated in panels C and D, were analyzed by LC-MS/MS. Translocon components identified in pPL45-Zn-163-containing samples (1b and 2b) are listed along with numbers of peptide matches, as compared to those identified in mock control (1a and 2a) or pPL-163-His (2c) purified RAMP samples. N1 and N2 are analyses of repeated experiments performed on different days. (F) Western blots confirmed complex C generated from pPL-137 and pPL45-Zn-163(+Zn+2) (ie pPL45-Zn-163 translated in the presence of Zn+2) contained Sec62 and Sec63. (G) Gel-shifts of complex C derived from pPL45-Zn-163(+Zn+2), pPL-163, and pPL-137 translations with indicated antibodies. See also Figure S4 – 6.
Figure 6. Sec62/63 is Associated with Complex C upon Initial Membrane Solubilization
(A) Schematic of RAMP1, 2, and 3 (R1, R2, and R3) isolation procedure consisting of one, two, or three rounds of RTC pelleting and resuspension before RAMP release. (B) Immunoblots of Sec63, Sec62, and Sec61 in RAMP fractions 1–3 that were generated by translation of the indicated nascent chains as compared to input (left lane). (C) Quantitation of immunoblots as in panel B. Percent of Sec63, Seec62, or Sec61 remaining in RAMP fractions 1–3 as compared to input is shown for indicated nascent chain translations ± SEM (n=3).(D) Immunoblots of RAMP1 fractions show location of Sec62 and Sec63 complexes on BN-PAGE with and without translated nascent chains (arrows and asterisks respectively).
Figure 7. PrP Stabilizes Sec62 and Sec63 RAMP Complexes
(A) A schematic of the prion protein (PrP) indicates location of signal sequence (SS), an N-terminal intrinsically disordered region, glycosylation sites, a GPI-anchor site, two helical domains, a pause-transfer sequence, and a TM segment. (B) SDS-PAGE analysis of translated PrP RTC intermediates ± CRMs. Peptidyl-tRNA was removed with RNase. (C) BN-PAGE of RAMPs prepared from indicated PrP and pPL translations. (D) Complex C isolation from PrP-167-His RAMP fractions by Ni-NTA purification after DTSSP crosslinking. (E) LC-MS/MS identified proteins from excised ~500 kD gel bands generated from mock or PrP-167-His Ni-NTA purified RAMP complexes as in (D). The asterisk denotes mock sample N1 that served as the control in a single experiment containing both pPL45-Zn-163-His (Figure 5E) and PrP-167-His samples. (F) Western blots of PrP RAMP fractions analyzed on BN- and SDS-PAGE. (G) Gel-shifts of PrP-167 RAMP complex C with indicated antibodies. (H) BN-PAGE of RAMPs prepared from indicated PrP truncations which lack the pause-transfer (PrP ΔPT) or TM segment (PrP ΔTM). (I) General model of dynamic translocon contacts (arrows) driven by nascent chain that could reflect repositioning of components within the RTC, recruitment of new factors, or affinity changes between bound components and/or polypeptide. See also Figure S5 and S7.
Similar articles
- Molecular view of ER membrane remodeling by the Sec61/TRAP translocon.
Karki S, Javanainen M, Rehan S, Tranter D, Kellosalo J, Huiskonen JT, Happonen L, Paavilainen V. Karki S, et al. EMBO Rep. 2023 Dec 6;24(12):e57910. doi: 10.15252/embr.202357910. Epub 2023 Nov 20. EMBO Rep. 2023. PMID: 37983950 Free PMC article. - Membrane translocation at the ER: with a little help from my friends.
O'Keefe S, High S. O'Keefe S, et al. FEBS J. 2020 Nov;287(21):4607-4611. doi: 10.1111/febs.15309. Epub 2020 Apr 16. FEBS J. 2020. PMID: 32301242 - Identification of signal peptide features for substrate specificity in human Sec62/Sec63-dependent ER protein import.
Schorr S, Nguyen D, Haßdenteufel S, Nagaraj N, Cavalié A, Greiner M, Weissgerber P, Loi M, Paton AW, Paton JC, Molinari M, Förster F, Dudek J, Lang S, Helms V, Zimmermann R. Schorr S, et al. FEBS J. 2020 Nov;287(21):4612-4640. doi: 10.1111/febs.15274. Epub 2020 Mar 20. FEBS J. 2020. PMID: 32133789 - Functions and Mechanisms of the Human Ribosome-Translocon Complex.
Lang S, Nguyen D, Pfeffer S, Förster F, Helms V, Zimmermann R. Lang S, et al. Subcell Biochem. 2019;93:83-141. doi: 10.1007/978-3-030-28151-9_4. Subcell Biochem. 2019. PMID: 31939150 Review. - Emerging View on the Molecular Functions of Sec62 and Sec63 in Protein Translocation.
Jung SJ, Kim H. Jung SJ, et al. Int J Mol Sci. 2021 Nov 25;22(23):12757. doi: 10.3390/ijms222312757. Int J Mol Sci. 2021. PMID: 34884562 Free PMC article. Review.
Cited by
- Asparagine-linked glycosylation is not directly coupled to protein translocation across the endoplasmic reticulum in Saccharomyces cerevisiae.
Shrimal S, Cherepanova NA, Mandon EC, Venev SV, Gilmore R. Shrimal S, et al. Mol Biol Cell. 2019 Oct 1;30(21):2626-2638. doi: 10.1091/mbc.E19-06-0330. Epub 2019 Aug 21. Mol Biol Cell. 2019. PMID: 31433728 Free PMC article. - Aberrant expression of Sec61α in esophageal cancers.
Bachmann K, Bockhorn M, Mann O, Gebauer F, Blessmann M, Izbicki JR, Grupp K. Bachmann K, et al. J Cancer Res Clin Oncol. 2019 Aug;145(8):2039-2044. doi: 10.1007/s00432-019-02955-7. Epub 2019 Jun 13. J Cancer Res Clin Oncol. 2019. PMID: 31197453 - Signal Peptide Features Determining the Substrate Specificities of Targeting and Translocation Components in Human ER Protein Import.
Lang S, Nguyen D, Bhadra P, Jung M, Helms V, Zimmermann R. Lang S, et al. Front Physiol. 2022 Jul 11;13:833540. doi: 10.3389/fphys.2022.833540. eCollection 2022. Front Physiol. 2022. PMID: 35899032 Free PMC article. Review. - Eat it right: ER-phagy and recovER-phagy.
Loi M, Fregno I, Guerra C, Molinari M. Loi M, et al. Biochem Soc Trans. 2018 Jun 19;46(3):699-706. doi: 10.1042/BST20170354. Epub 2018 May 25. Biochem Soc Trans. 2018. PMID: 29802216 Free PMC article. Review. - Chaperone-Mediated Sec61 Channel Gating during ER Import of Small Precursor Proteins Overcomes Sec61 Inhibitor-Reinforced Energy Barrier.
Haßdenteufel S, Johnson N, Paton AW, Paton JC, High S, Zimmermann R. Haßdenteufel S, et al. Cell Rep. 2018 May 1;23(5):1373-1386. doi: 10.1016/j.celrep.2018.03.122. Cell Rep. 2018. PMID: 29719251 Free PMC article.
References
- Crowley KS, Liao S, Worrell VE, Reinhart GD, Johnson AE. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 1994;78:461–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM53457/GM/NIGMS NIH HHS/United States
- P30EY010572/EY/NEI NIH HHS/United States
- P30 EY010572/EY/NEI NIH HHS/United States
- DK51818/DK/NIDDK NIH HHS/United States
- S10OD012246/OD/NIH HHS/United States
- T32 HL083808/HL/NHLBI NIH HHS/United States
- S10 OD012246/OD/NIH HHS/United States
- F32 GM083568/GM/NIGMS NIH HHS/United States
- R01 DK051818/DK/NIDDK NIH HHS/United States
- P30CA069533/CA/NCI NIH HHS/United States
- R01 GM053457/GM/NIGMS NIH HHS/United States
- P30 CA069533/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources