Addition of a Gastrointestinal Microbiome Modulator to Metformin Improves Metformin Tolerance and Fasting Glucose Levels - PubMed (original) (raw)
Randomized Controlled Trial
Addition of a Gastrointestinal Microbiome Modulator to Metformin Improves Metformin Tolerance and Fasting Glucose Levels
Jeffrey H Burton et al. J Diabetes Sci Technol. 2015 Jul.
Abstract
Background: Adverse effects of metformin are primarily related to gastrointestinal (GI) intolerance that could limit titration to an efficacious dose or cause discontinuation of the medication. Because some metformin side effects may be attributable to shifts in the GI microbiome, we tested whether a GI microbiome modulator (GIMM) used in combination with metformin would ameliorate the GI symptoms.
Methods: A 2-period crossover study design was used with 2 treatment sequences, either placebo in period 1 followed by GIMM in period 2 or vice versa. Study periods lasted for 2 weeks, with a 2-week washout period between. During the first week, type 2 diabetes patients (T2D) who experienced metformin GI intolerance took 500 mg metformin along with their assigned NM504 (GIMM) or placebo treatment with breakfast and with dinner. In the second week, the 10 subjects took 500 mg metformin (t.i.d.), with GIMM or placebo consumed with the first and third daily metformin doses. Subjects were permitted to discontinue metformin dosing if it became intolerable.
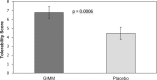
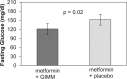
Results: The combination of metformin and GIMM treatment produced a significantly better tolerance score to metformin than the placebo combination (6.78 ± 0.65 [mean ± SEM] versus 4.45 ± 0.69, P = .0006). Mean fasting glucose levels were significantly (P < .02) lower with the metformin-GIMM combination (121.3 ± 7.8 mg/dl) than with metformin-placebo (151.9 ± 7.8 mg/dl).
Conclusion: Combining a GI microbiome modulator with metformin might allow the greater use of metformin in T2D patients and improve treatment of the disease.
Keywords: GI adverse events; NM504; glucose; metformin; metformin intolerance; microbiome modulator.
© 2015 Diabetes Technology Society.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FLG is a MicroBiome Therapeutics clinical advisor. MLH is a full-time employee of MicroBiome Therapeutics.
Figures
Figure 1.
Metformin is better tolerated when combined with GIMM than placebo. Tolerability is a composite score of GI symptoms. A greater tolerability score indicates a better metformin tolerance.
Figure 2.
Fasting blood glucose levels. Subjects were assigned GIMM (A) or placebo (B) for the initial period. Subjects were instructed to take 500 mg metformin b.i.d. during the initial week of each period and 500 mg metformin t.i.d. during the second week. Subjects were permitted to discontinue metformin dosing if symptoms became intolerable. Days metformin was taken are indicated by the solid symbols. Subject number is indicated in the upper left as well as in Table 1.
Figure 3.
Mean observed fasting glucose for subjects taking GIMM during period 1. Bars represent the mean ± SEM.
Similar articles
- Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: A randomized controlled pilot trial.
Rebello CJ, Burton J, Heiman M, Greenway FL. Rebello CJ, et al. J Diabetes Complications. 2015 Nov-Dec;29(8):1272-6. doi: 10.1016/j.jdiacomp.2015.08.023. Epub 2015 Sep 3. J Diabetes Complications. 2015. PMID: 26424589 Free PMC article. Clinical Trial. - Glucose and lipid effects of the ileal apical sodium-dependent bile acid transporter inhibitor GSK2330672: double-blind randomized trials with type 2 diabetes subjects taking metformin.
Nunez DJ, Yao X, Lin J, Walker A, Zuo P, Webster L, Krug-Gourley S, Zamek-Gliszczynski MJ, Gillmor DS, Johnson SL. Nunez DJ, et al. Diabetes Obes Metab. 2016 Jul;18(7):654-62. doi: 10.1111/dom.12656. Epub 2016 Apr 21. Diabetes Obes Metab. 2016. PMID: 26939572 Clinical Trial. - Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.
Plosker GL, Figgitt DP. Plosker GL, et al. Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review. - Metformin and gut microbiota: their interactions and their impact on diabetes.
Vallianou NG, Stratigou T, Tsagarakis S. Vallianou NG, et al. Hormones (Athens). 2019 Jun;18(2):141-144. doi: 10.1007/s42000-019-00093-w. Epub 2019 Feb 4. Hormones (Athens). 2019. PMID: 30719628 Review.
Cited by
- Targeting the Intestinal Microbiota to Prevent Type 2 Diabetes and Enhance the Effect of Metformin on Glycaemia: A Randomised Controlled Pilot Study.
Palacios T, Vitetta L, Coulson S, Madigan CD, Lam YY, Manuel R, Briskey D, Hendy C, Kim JN, Ishoey T, Soto-Giron MJ, Schott EM, Toledo G, Caterson ID. Palacios T, et al. Nutrients. 2020 Jul 9;12(7):2041. doi: 10.3390/nu12072041. Nutrients. 2020. PMID: 32660025 Free PMC article. Clinical Trial. - Apoptotic and proliferative processes in the small intestine of rats with type 2 diabetes mellitus after metformin and propionic acid treatment.
Natrus L, Lisakovska O, Smirnov A, Osadchuk Y, Klys Y. Natrus L, et al. Front Pharmacol. 2024 Oct 16;15:1477793. doi: 10.3389/fphar.2024.1477793. eCollection 2024. Front Pharmacol. 2024. PMID: 39478962 Free PMC article. - Microbiome differences related to metformin intolerance among Black individuals with diabetes, a pilot cross-sectional study.
Fayfman M, Gewirtz AT, Delaroque C, Blanco G, Gibanica S, Srinivasan S, Chassaing B. Fayfman M, et al. Metabol Open. 2023 Sep 15;20:100256. doi: 10.1016/j.metop.2023.100256. eCollection 2023 Dec. Metabol Open. 2023. PMID: 38115865 Free PMC article. - Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK.
Li S, Zhang Y, Sun Y, Zhang G, Bai J, Guo J, Su X, Du H, Cao X, Yang J, Wang T. Li S, et al. Nutr Diabetes. 2019 Oct 7;9(1):28. doi: 10.1038/s41387-019-0095-8. Nutr Diabetes. 2019. PMID: 31591391 Free PMC article. - Metformin in Combination with Malvidin Prevents Progression of Non-Alcoholic Fatty Liver Disease via Improving Lipid and Glucose Metabolisms, and Inhibiting Inflammation in Type 2 Diabetes Rats.
Zou W, Zhang C, Gu X, Li X, Zhu H. Zou W, et al. Drug Des Devel Ther. 2021 Jun 17;15:2565-2576. doi: 10.2147/DDDT.S307257. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34168429 Free PMC article.
References
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2015; 38:140-149. - PubMed
- Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81-98. - PubMed
- Florez H, Luo J, Castillo-Florez S, et al. Impact of metformin-induced gastrointestinal symptoms on quality of life and adherence in patients with type 2 diabetes. Postgrad Med. 2010;122:112-120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1 U54 GM104940/GM/NIGMS NIH HHS/United States
- 2P30DK072476/DK/NIDDK NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- T35 DK093428/DK/NIDDK NIH HHS/United States
- P50 AT002776/AT/NCCIH NIH HHS/United States
- P50AT002776/AT/NCCIH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical