Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis - PubMed (original) (raw)
Review
Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis
Lars B Holst et al. BMJ. 2015.
Abstract
Objective: To compare the benefit and harm of restrictive versus liberal transfusion strategies to guide red blood cell transfusions.
Design: Systematic review with meta-analyses and trial sequential analyses of randomised clinical trials.
Data sources: Cochrane central register of controlled trials, SilverPlatter Medline (1950 to date), SilverPlatter Embase (1980 to date), and Science Citation Index Expanded (1900 to present). Reference lists of identified trials and other systematic reviews were assessed, and authors and experts in transfusion were contacted to identify additional trials.
Trial selection: Published and unpublished randomised clinical trials that evaluated a restrictive compared with a liberal transfusion strategy in adults or children, irrespective of language, blinding procedure, publication status, or sample size.
Data extraction: Two authors independently screened titles and abstracts of trials identified, and relevant trials were evaluated in full text for eligibility. Two reviewers then independently extracted data on methods, interventions, outcomes, and risk of bias from included trials. random effects models were used to estimate risk ratios and mean differences with 95% confidence intervals.
Results: 31 trials totalling 9813 randomised patients were included. The proportion of patients receiving red blood cells (relative risk 0.54, 95% confidence interval 0.47 to 0.63, 8923 patients, 24 trials) and the number of red blood cell units transfused (mean difference -1.43, 95% confidence interval -2.01 to -0.86) were lower with the restrictive compared with liberal transfusion strategies. Restrictive compared with liberal transfusion strategies were not associated with risk of death (0.86, 0.74 to 1.01, 5707 patients, nine lower risk of bias trials), overall morbidity (0.98, 0.85 to 1.12, 4517 patients, six lower risk of bias trials), or fatal or non-fatal myocardial infarction (1.28, 0.66 to 2.49, 4730 patients, seven lower risk of bias trials). Results were not affected by the inclusion of trials with unclear or high risk of bias. Using trial sequential analyses on mortality and myocardial infarction, the required information size was not reached, but a 15% relative risk reduction or increase in overall morbidity with restrictive transfusion strategies could be excluded.
Conclusions: Compared with liberal strategies, restrictive transfusion strategies were associated with a reduction in the number of red blood cell units transfused and number of patients being transfused, but mortality, overall morbidity, and myocardial infarction seemed to be unaltered. Restrictive transfusion strategies are safe in most clinical settings. Liberal transfusion strategies have not been shown to convey any benefit to patients.
Trial registration: PROSPERO CRD42013004272.
© Holst et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare: AP was principal investigator for Transfusion Requirements In Septic Shock (TRISS) trial and LBH, NH, and JW were members of the steering committee. AP is head of research in his intensive care unit, which receives research grants from CSL Behring, Fresenius Kabi, and Cosmed.
Figures
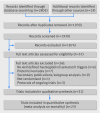
Fig 1 Flow of trials through study
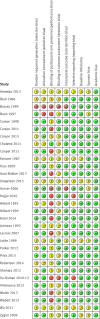
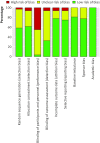
Fig 2 Risk of bias summary for all included records
Fig 3 Risk of bias graph
Fig 4 Forest plot of mortality in lower risk of bias trials. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
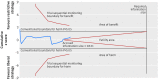
Fig 5 Trial sequential analysis of nine trials with lower risk of bias reporting all cause mortality, control event proportion of 17.4%, diversity of 56%, α of 5%, power of 80%, and relative risk reduction of 15%. The required information size of 14 217 has not been reached and none of the boundaries for benefit, harm, or futility has been crossed, leaving the meta-analysis inconclusive of a 15% relative risk reduction. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 0.86 is 0.67 to 1.12
Fig 6 Forest plot of mortality despite risk of bias. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
Fig 7 Trial sequential analysis of 23 trials (despite risk of bias) reporting mortality, with control event proportion of 13.7%, diversity of 62%, α of 5%, power of 80%, and relative risk reduction of 15%. The required information size of 20 799 is far from reached and none of the boundaries for benefit, harm, or futility has been crossed, leaving the meta-analysis inconclusive of a 15% relative risk reduction. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 0.95 is 0.74 to 1.21
Fig 8 Forest plot of mortality in trials stratified by clinical setting. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
Fig 9 Forest plot of overall morbidity in low risk of bias trials. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
Fig 10 Trial sequential analysis of six trials reporting overall morbidity, a control event proportion of 40%, diversity of 75%, α of 5%, power of 80%, and relative risk reduction of 15%. The required information size of 7188 has not been reached, but the boundaries for futility are crossed, leaving out the possibility of a 15% relative risk reduction. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 0.98 is 0.81 to 1.19
Fig 11 Forest plot of myocardial infarctions in low risk of bias trials. Size of squares for risk ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals
Fig 12 Trial sequential analysis of seven trials reporting myocardial infarction, with a control event proportion of 1.8%, diversity of 62.3%, α of 5%, power of 80%, and relative risk reduction of 50%. The diversity adjusted required information size of 13 686 is far from reached and none of the boundaries for benefit, harm, or futility has been crossed, leaving the meta-analysis inconclusive of even a 50% relative risk reduction. The trial sequential analysis adjusted 95% confidence interval for a relative risk of 1.28 is 0.40 to 4.13
Comment in
- Red cell transfusions for treating anaemia in the absence of bleeding.
Walsh TS. Walsh TS. BMJ. 2015 Mar 24;350:h1463. doi: 10.1136/bmj.h1463. BMJ. 2015. PMID: 25804542 No abstract available. - Restrictive red blood cell transfusion strategies appear safe in most clinical settings.
Roubinian NH, Carson JL. Roubinian NH, et al. Evid Based Med. 2015 Oct;20(5):170. doi: 10.1136/ebmed-2015-110218. Epub 2015 Jul 28. Evid Based Med. 2015. PMID: 26220955 No abstract available.
Similar articles
- Transfusion thresholds for guiding red blood cell transfusion.
Carson JL, Stanworth SJ, Dennis JA, Trivella M, Roubinian N, Fergusson DA, Triulzi D, Dorée C, Hébert PC. Carson JL, et al. Cochrane Database Syst Rev. 2021 Dec 21;12(12):CD002042. doi: 10.1002/14651858.CD002042.pub5. Cochrane Database Syst Rev. 2021. PMID: 34932836 Free PMC article. Review. - Red blood cell transfusion for people undergoing hip fracture surgery.
Brunskill SJ, Millette SL, Shokoohi A, Pulford EC, Doree C, Murphy MF, Stanworth S. Brunskill SJ, et al. Cochrane Database Syst Rev. 2015 Apr 21;2015(4):CD009699. doi: 10.1002/14651858.CD009699.pub2. Cochrane Database Syst Rev. 2015. PMID: 25897628 Free PMC article. Review. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion.
Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, Hebert PC. Carson JL, et al. Cochrane Database Syst Rev. 2016 Oct 12;10(10):CD002042. doi: 10.1002/14651858.CD002042.pub4. Cochrane Database Syst Rev. 2016. PMID: 27731885 Free PMC article. Updated. Review.
Cited by
- Patient blood management: A solution for South Africa.
Thomson J, Hofmann A, Barrett CA, Beeton A, Bellairs GRM, Boretti L, Coetzee MJ, Farmer S, Gibbs MW, H Gombotz H, Hilton C, Kassianides C, Louw VJ, Lundgren C, Mahlangu JN, Noel CB, Rambiritch V, Schneider F, Verburgh E, Wessels PL, Wessels P, Wise R, Shander On Behalf Of The South African Patient Blood Management Group A. Thomson J, et al. S Afr Med J. 2019 Jun 28;109(7):471-476. doi: 10.7196/SAMJ.2019.v109i7.13859. S Afr Med J. 2019. PMID: 31266571 Free PMC article. - A Systematic Review and Meta-Analysis of the Clinical Appropriateness of Blood Transfusion in China.
Zhu C, Gao Y, Li Z, Li Q, Gao Z, Liao Y, Deng Z. Zhu C, et al. Medicine (Baltimore). 2015 Dec;94(50):e2164. doi: 10.1097/MD.0000000000002164. Medicine (Baltimore). 2015. PMID: 26683925 Free PMC article. Review. - Restrictive versus liberal red blood cell transfusion strategies for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support.
Estcourt LJ, Malouf R, Trivella M, Fergusson DA, Hopewell S, Murphy MF. Estcourt LJ, et al. Cochrane Database Syst Rev. 2017 Jan 27;1(1):CD011305. doi: 10.1002/14651858.CD011305.pub2. Cochrane Database Syst Rev. 2017. PMID: 28128441 Free PMC article. Updated. Review. - Restrictive versus liberal transfusion strategies for red blood cell transfusion after hip or knee surgery: A systematic review and meta-analysis.
Mao T, Gao F, Han J, Sun W, Guo W, Li Z, Wang W. Mao T, et al. Medicine (Baltimore). 2017 Jun;96(25):e7326. doi: 10.1097/MD.0000000000007326. Medicine (Baltimore). 2017. PMID: 28640148 Free PMC article. Review. - A Mendelian Randomization Study of Plasma Homocysteine and Multiple Myeloma.
Xuan Y, Li XH, Hu ZQ, Teng ZM, Hu DJ. Xuan Y, et al. Sci Rep. 2016 Apr 29;6:25204. doi: 10.1038/srep25204. Sci Rep. 2016. PMID: 27126524 Free PMC article.
References
- Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, et al. The CRIT Study: anemia and blood transfusion in the critically ill-current clinical practice in the United States. Crit Care Med 2004;32:39-52. - PubMed
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344-53. - PubMed
- Hébert P, Wells G, Blajchman MA, Marshall JC. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999;340:409-17. - PubMed
- Lacroix J, Hébert PC, Hutchison JS, Hume HA. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007;356:1609-19. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources