PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation - PubMed (original) (raw)
Kankana Bardhan 1, Pranam Chatterjee 1, Duygu Sari 1, Bianling Liu 1, Lauren N Bell 2, Edward D Karoly 2, Gordon J Freeman 3, Victoria Petkova 1, Pankaj Seth 4, Lequn Li 1, Vassiliki A Boussiotis 1
Affiliations
- PMID: 25809635
- PMCID: PMC4389235
- DOI: 10.1038/ncomms7692
PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation
Nikolaos Patsoukis et al. Nat Commun. 2015.
Abstract
During activation, T cells undergo metabolic reprogramming, which imprints distinct functional fates. We determined that on PD-1 ligation, activated T cells are unable to engage in glycolysis or amino acid metabolism but have an increased rate of fatty acid β-oxidation (FAO). PD-1 promotes FAO of endogenous lipids by increasing expression of CPT1A, and inducing lipolysis as indicated by elevation of the lipase ATGL, the lipolysis marker glycerol and release of fatty acids. Conversely, CTLA-4 inhibits glycolysis without augmenting FAO, suggesting that CTLA-4 sustains the metabolic profile of non-activated cells. Because T cells utilize glycolysis during differentiation to effectors, our findings reveal a metabolic mechanism responsible for PD-1-mediated blockade of T-effector cell differentiation. The enhancement of FAO provides a mechanistic explanation for the longevity of T cells receiving PD-1 signals in patients with chronic infections and cancer, and for their capacity to be reinvigorated by PD-1 blockade.
Conflict of interest statement
G.J.F. receives royalties from patents related to PD-1 pathway. The other authors declare that they have no competing interests.
Figures
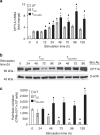
Figure 1. PD-1 inhibits transport and utilization of glucose during T-cell activation.
CD4+ primary human T cells were either left unstimulated (UT) or were incubated with magnetic beads conjugated with αCD3/αCD28/IgG (T cells co-stimulated (TCC)) or magnetic beads conjugated with αCD3/αCD28/PD-L1-Ig fusion protein (T cells co-stimulated+PD-1 (TCC+PD1)). (a) IFN-γ production was assessed by ELISA (*P<0.05 TCC+PD1 versus TCC; _n_=3 experiments; Student’s _t_-test). (b,c) Expression of Glut1 after culture under the indicated conditions and time intervals was examined by flow cytometry, and glucose uptake was examined by 2-{14C(U)}-deoxy-D-glucose incorporation. At each time point glucose uptake in TCC+PD1-stimulated cells was compared with TCC-stimulated cells (*P<0.05, TCC+PD1 versus TCC; _n_=3 experiments; Student’s _t_-test). (d) Analysis of key metabolites involved in glycolysis was performed in cells and culture supernatants as described in Methods. The amounts of the indicated metabolites in unstimulated, TCC and TCC+PD1 cells were plotted in whisker boxes. The lower and upper sides of the box indicate the first and third quartile, respectively. The horizontal line inside the box indicates the median value, whereas the lower and upper bars indicate the minimum and maximum of distribution, respectively; (+) mean value; (o) extreme data point. Results of five measurements generated from five independent experiments (*P<0.05, TCC+PD1 versus TCC, Welch’s t_-test; ♦_P<0.05, TCC versus UT and TCC+PD1 versus UT, analysis of variance). (e) HK2 mRNA was analysed by real-time quantitative PCR and relative expression of mRNA of each time point and culture condition over the levels expressed in unstimulated cells (defined as 1) is shown. Analysis was performed over prolonged time of culture (120 h) to investigate whether quantitative difference versus altered kinetics of HK2 expression was induced by PD-1 (*P<0.05, TCC+PD1 versus TCC, Student’s _t_-test; _n_=3 experiments).
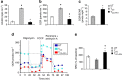
Figure 2. PD-1 inhibits transport and catabolism of glutamine and branched-chain amino acids during T-cell activation.
(a) SNAT1 and SNAT2 mRNA was analysed by real-time quantitative PCR and relative expression of mRNA of each time point and culture condition over the levels expressed in unstimulated cells (defined as 1) is shown (*P<0.05, TCC+PD1 versus TCC, Student’s _t_-test; _n_=3 experiments). (b) The amounts of glutamine and the glutaminolysis metabolite, glutamate, valine and the metabolic product of valine catabolism, 4-methyl-2-oxopentanoate, were analysed in UT, TCC and TCC+PD1 cells and their culture supernatants were plotted in whisker boxes (*P<0.05, TCC+PD1 versus TCC, Welch’s t_-test; ♦_P<0.05, TCC+PD1 versus UT and TCC versus UT, analysis of variance). Results of five replicate samples generated from five independent experiments. (c) Mitochondrial membrane potential (ΔΨm) was assessed after the indicated time intervals of culture by staining with the potentiometric dye TMRE and analysis by flow cytometry; mean fluorescence intensity (MFI) of each sample is shown in the tables. Results are representative of three experiments.
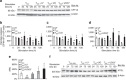
Figure 3. PD-1 induces upregulation of CPT1A and fatty acid β-oxidation.
(a) CPT1A mRNA was analysed by real-time quantitative PCR and relative expression of mRNA of each time point and culture condition over the levels expressed in unstimulated cells (defined as 1) is shown (*P<0.05, TCC+PD1 versus TCC, Student’s t_-test; ♦_P<0.05, TCC+PD1 versus UT and TCC versus UT, analysis of variance (ANOVA); n_=3 experiments). (b) CD4+ primary human T cells were cultured under the indicated conditions. Cell lysates were prepared at the indicated time points and expression of CPT1A and β-actin were assessed by SDS–PAGE and immunoblot. Results are representative of three experiments. (c) Fatty acid β-oxidation rate after culture under the indicated conditions for various time intervals was examined. Values of TCC and TCC+PD1 cells were compared with unstimulated (UT) cells (♦_P<0.05, ANOVA; _n_=3) and values of TCC+PD1 were compared with TCC cells (*P<0.05, Student’s _t_-test; _n_=3).
Figure 4. PD-1 induces lipolysis and utilization of endogenous fatty acids for β-oxidation.
(a) The amounts of n3DPA;22:5n3 and glycerol-3-phosphate (G3P) in the cells and the amounts of DHA;22:6n3 and 3-hyroxybutyrate (BHBA) in the culture supernatants of the indicated culture conditions were analysed and values were plotted in whisker boxes. Values of TCC and TCC+PD1 cells were compared with unstimulated (UT) cells (♦P<0.05, analysis of variance (ANOVA); _n_=5) and values of TCC+PD1 were compared with TCC cells (*P<0.05, Welch _t_-test; n_=5). (b) Purified human T cells were cultured under the indicated conditions. Cell lysates were prepared at the indicated time points and expression of FASN, ATGL and β-actin were assessed by SDS–PAGE and immunoblot. Results are representative of three experiments. (c) ATGL mRNA was analysed by real-time quantitative PCR and relative expression of mRNA of each time point and culture condition over the levels expressed in unstimulated (UT) cells (defined as 1) is shown. Expression levels of ATGL mRNA in TCC+PD1-stimulated cells were compared with TCC-stimulated cells (*P<0.05, Student’s t_-test) and expression levels in TCC and TCC+PD1-stimulated cells were compared with unstimulated (UT) cells (♦_P<0.05, ANOVA). Results are mean±s.e.m. of three experiments. (d) T cells were cultured with [9,10-3H]palmitate for 48 h, over which time the radiolabelled fatty acid was used to generate cellular lipids. Subsequently, cells were washed to remove unincorporated label and were cultured with beads conjugated with aCD3/CD28/IgG or with beads conjugated with aCD3/CD28/PD-L1-Ig and the amount of β-oxidation of labelled fatty acids generated from endogenous lipid was determined each day. Values of TCC and TCC+PD1 cells were compared with unstimulated (UT) cells (♦_P<0.05, ANOVA; _n_=3) and values of TCC+PD1 were compared with TCC cells (*P<0.05, Student’s _t_-test; _n_=3).
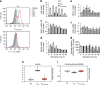
Figure 5. T cells receiving PD-1 signals have substantial mitochondrial spare respiratory capacity.
(a,b) CD4+ primary human T cells were cultured under the indicated conditions and 72 h of culture extracellular acidification rate (ECAR) and oxygen consumption rates (OCR) were assessed. (c) OCR/ECAR ratio was also measured. (d) OCR for each group was measured in real time under basal conditions and after addition of the indicated mitochondrial inhibitors. (e) Spare respiratory capacity (SRC) indicated by the difference of maximum OCR over basal OCR (arrows in d) calculated as percentage of basal OCR was also determined. Values of TCC and TCC+PD1 cells were compared with unstimulated (UT) cells (♦P<0.05, analysis of variance; _n_=4); values of TCC+PD1 cells were compared with TCC cells (*P<0.05, Student’s _t_-test; _n_=4).
Figure 6. Inhibition of PI3K/Akt and MEK/Erk pathways is involved in the metabolic reprogramming of activated T cells.
(a) CD4+ primary human T cells were cultured with tosyl-activated magnetic beads conjugated with aCD3/aCD28/IgG (TCC) in the presence of either LY294002 (LY, 10 μM), UO126 (UO, 10 μM) or their combination. Cell lysates were prepared at the indicated time points and expression of CPT1A and β-actin was assessed by SDS–PAGE and immunoblot. Results are representative of three experiments. (b–d) In parallel experiments, rate of fatty acid β-oxidation was examined. Values of TCC+inhibitor cultures were compared with TCC (*P<0.05, Student’s _t_-test; n_=3) and values of TCC and TCC+inhibitor cultures were compared with unstimulated (UT) (♦_P<0.05, analysis of variance (ANOVA); n_=3). (e) At 72 h of culture under the indicated conditions, spare respiratory capacity (SRC) was determined. Values of TCC and TCC+inhibitor cells were compared with unstimulated (UT) cells (♦_P<0.05, ANOVA; _n_=3) and values in TCC+inhibitor cells were compared with TCC (*P<0.05, Student’s _t_-test; _n_=3). (f) T cells were cultured with tosyl-activated magnetic beads conjugated with aCD3/aCD28/PD-L1-Ig or with tosylatcivated magnetic beads conjugated with aCD3/aCD28/IgG in the presence of either LY294002 (LY, 10 μM), UO126 (UO, 10 μM) or their combination. Cell lysates were prepared at the indicated time points and expression of ATGL and β-actin was assessed by SDS–PAGE and immunoblot.
Figure 7. CTLA-4 inhibits glycolytic reprogramming without increasing the rate of fatty acid β-oxidation.
CD4+ primary human T cells were either left unstimulated (UT) or were incubated with magnetic beads conjugated with αCD3/αCD28/IgG (T cells co-stimulated (TCC)) or magnetic beads conjugated with αCD3/αCD28/αCTLA-4 mAbs (T cells co-stimulated+ CTLA-4 (TCC+CTLA4)). (a) Expression of Glut1 after culture under the indicated conditions and time intervals was examined by flow cytometry. Results are representative of three experiments. (b–f) mRNA for HK2, SNAT1, SNAT2, CPT1A and ATGL was analysed by real-time quantitative PCR and relative expression of mRNA of each time point and culture condition over the levels expressed in unstimulated cells (defined as 1) is shown. (g) Fatty acid β-oxidation rate after culture under the indicated conditions for various time intervals was examined. (h,i) Analysis of lactate (h) and 3-hyroxybutyrate (i), end metabolites of glycolysis and fatty acid β-oxidation, respectively, was performed in culture supernatants. (For the studies shown in all panels: *P<0.05, TCC+CTLA4 versus TCC, Student’s t_-test; ♦_P<0.05, TCC versus UT and TCC+CTLA4 versus UT, analysis of variance; _n_=3 experiments).
Figure 8. PD-1 alters the metabolic programme of activated T cells from glycolysis to FAO.
CD4+ human T cells were pre-activated with anti-CD3-and-anti-CD28 mAbs for 4 h or for 24 h and subsequently were collected, were left to rest for 3 h and re-cultured with tosyl-activated magnetic beads conjugated with αCD3/αCD28/PD-L1-Ig (Tpr4hr→PD-1 and Tpr24hr→PD-1). In the same experiment CD4+ T cells without pre-activation were stimulated with tosyl-activated magnetic beads conjugated with αCD3/αCD28/IgG (TCC) and used as positive control for optimal stimulation without PD-1. (a) Expression of Glut1 after culture under the indicated conditions and time intervals was examined by flow cytometry. (b,c) mRNA for HK2 and CPT1A was analysed by real-time quantitative PCR and relative expression of mRNA of each time point and culture condition over the levels expressed in unstimulated cells (defined as 1) is shown. (d) Fatty acid β-oxidation rate after culture under the indicated conditions for various time intervals was examined. (e,f) Analysis of lactate (e) and 3-hydroxybutyrate (f), end metabolites of glycolysis and fatty acid β-oxidation, respectively, was performed in culture supernatants (for the studies shown in all panels: *P<0.05, Tpr4hr→PD-1 versus TCC or Tpr4hr→PD-1 versus TCC, Student’s t_-test; ♦_P<0.05, TCC versus UT, Tpr4hr→PD-1 versus UT and Tpr4hr→PD-1 versus UT, analysis of variance; _n_=3 experiments).
Similar articles
- STAT3 Activation-Induced Fatty Acid Oxidation in CD8+ T Effector Cells Is Critical for Obesity-Promoted Breast Tumor Growth.
Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T, Zhang Z, Li W, Lee H, Aftabizadeh M, Li YJ, Lee PP, Forman S, Somlo G, Chu P, Kruper L, Mortimer J, Hoon DSB, Huang W, Priceman S, Yu H. Zhang C, et al. Cell Metab. 2020 Jan 7;31(1):148-161.e5. doi: 10.1016/j.cmet.2019.10.013. Epub 2019 Nov 21. Cell Metab. 2020. PMID: 31761565 Free PMC article. - Etomoxir Actions on Regulatory and Memory T Cells Are Independent of Cpt1a-Mediated Fatty Acid Oxidation.
Raud B, Roy DG, Divakaruni AS, Tarasenko TN, Franke R, Ma EH, Samborska B, Hsieh WY, Wong AH, Stüve P, Arnold-Schrauf C, Guderian M, Lochner M, Rampertaap S, Romito K, Monsale J, Brönstrup M, Bensinger SJ, Murphy AN, McGuire PJ, Jones RG, Sparwasser T, Berod L. Raud B, et al. Cell Metab. 2018 Sep 4;28(3):504-515.e7. doi: 10.1016/j.cmet.2018.06.002. Epub 2018 Jun 28. Cell Metab. 2018. PMID: 30043753 Free PMC article. - IDO decreases glycolysis and glutaminolysis by activating GCN2K, while it increases fatty acid oxidation by activating AhR, thus preserving CD4+ T‑cell survival and proliferation.
Eleftheriadis T, Pissas G, Liakopoulos V, Stefanidis I. Eleftheriadis T, et al. Int J Mol Med. 2018 Jul;42(1):557-568. doi: 10.3892/ijmm.2018.3624. Epub 2018 Apr 16. Int J Mol Med. 2018. PMID: 29693118 - Fatty acid oxidation: An emerging facet of metabolic transformation in cancer.
Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang W, Wang XY, Fang X. Ma Y, et al. Cancer Lett. 2018 Oct 28;435:92-100. doi: 10.1016/j.canlet.2018.08.006. Epub 2018 Aug 10. Cancer Lett. 2018. PMID: 30102953 Free PMC article. Review. - Rethinking the paradigm: How comparative studies on fatty acid oxidation inform our understanding of T cell metabolism.
Chiaranunt P, Ferrara JL, Byersdorfer CA. Chiaranunt P, et al. Mol Immunol. 2015 Dec;68(2 Pt C):564-74. doi: 10.1016/j.molimm.2015.07.023. Epub 2015 Sep 8. Mol Immunol. 2015. PMID: 26359186 Free PMC article. Review.
Cited by
- Metabolic dialogues: regulators of chimeric antigen receptor T cell function in the tumor microenvironment.
Moraly J, Kondo T, Benzaoui M, DuSold J, Talluri S, Pouzolles MC, Chien C, Dardalhon V, Taylor N. Moraly J, et al. Mol Oncol. 2024 Jul;18(7):1695-1718. doi: 10.1002/1878-0261.13691. Epub 2024 Jun 22. Mol Oncol. 2024. PMID: 38922759 Free PMC article. Review. - Energy demanding RNA and protein metabolism drive dysfunctionality of HIV-specific T cell changes during chronic HIV infection.
van Pul L, Stunnenberg M, Kroeze S, van Dort KA, Boeser-Nunnink BDM, Harskamp AM, Geijtenbeek TBH, Kootstra NA. van Pul L, et al. PLoS One. 2024 Oct 2;19(10):e0298472. doi: 10.1371/journal.pone.0298472. eCollection 2024. PLoS One. 2024. PMID: 39356699 Free PMC article. - Mechanisms of Altered Immune Response in Skin Melanoma.
Fruntealată RF, Marius M, Boboc IKS, Mitran SI, Ciurea ME, Stoica GA. Fruntealată RF, et al. Curr Health Sci J. 2023 Jul-Sep;49(3):297-311. doi: 10.12865/CHSJ.49.03.01. Epub 2023 Sep 30. Curr Health Sci J. 2023. PMID: 38314217 Free PMC article. Review. - Immune metabolism in PD-1 blockade-based cancer immunotherapy.
Kumar A, Chamoto K. Kumar A, et al. Int Immunol. 2021 Jan 1;33(1):17-26. doi: 10.1093/intimm/dxaa046. Int Immunol. 2021. PMID: 32622347 Free PMC article. Review. - Metabolic communication in the tumour-immune microenvironment.
Kao KC, Vilbois S, Tsai CH, Ho PC. Kao KC, et al. Nat Cell Biol. 2022 Nov;24(11):1574-1583. doi: 10.1038/s41556-022-01002-x. Epub 2022 Oct 13. Nat Cell Biol. 2022. PMID: 36229606 Review.
References
- Probst H. C., McCoy K., Okazaki T., Honjo T. & van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 6, 280–286 (2005) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI098129/AI/NIAID NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- R01 CA183605/CA/NCI NIH HHS/United States
- R56 AI098129/AI/NIAID NIH HHS/United States
- CA183605/CA/NCI NIH HHS/United States
- AI056299/AI/NIAID NIH HHS/United States
- HHSN2722018C/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials