Preservation of the blood brain barrier and cortical neuronal tissue by liraglutide, a long acting glucagon-like-1 analogue, after experimental traumatic brain injury - PubMed (original) (raw)
Preservation of the blood brain barrier and cortical neuronal tissue by liraglutide, a long acting glucagon-like-1 analogue, after experimental traumatic brain injury
Jakob Hakon et al. PLoS One. 2015.
Abstract
Cerebral edema is a common complication following moderate and severe traumatic brain injury (TBI), and a significant risk factor for development of neuronal death and deterioration of neurological outcome. To this date, medical approaches that effectively alleviate cerebral edema and neuronal death after TBI are not available. Glucagon-like peptide-1 (GLP-1) has anti-inflammatory properties on cerebral endothelium and exerts neuroprotective effects. Here, we investigated the effects of GLP-1 on secondary injury after moderate and severe TBI. Male Sprague Dawley rats were subjected either to TBI by Controlled Cortical Impact (CCI) or sham surgery. After surgery, vehicle or a GLP-1 analogue, Liraglutide, were administered subcutaneously twice daily for two days. Treatment with Liraglutide (200 μg/kg) significantly reduced cerebral edema in pericontusional regions and improved sensorimotor function 48 hours after CCI. The integrity of the blood-brain barrier was markedly preserved in Liraglutide treated animals, as determined by cerebral extravasation of Evans blue conjugated albumin. Furthermore, Liraglutide reduced cortical tissue loss, but did not affect tissue loss and delayed neuronal death in the thalamus on day 7 post injury. Together, our data suggest that the GLP-1 pathway might be a promising target in the therapy of cerebral edema and cortical neuronal injury after moderate and severe TBI.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Fig 1. Experimental outline.
Flow diagram of the three experiments. The colored dots indicate treatment with a subcutaneous dose of Liraglutide 75 μg/kg (LIR75), Liraglutide 200 μg/kg (LIR200) or vehicle. Abbreviations: Controlled cortical impact (CCI), days (d), experiment (exp), Paw-placement test (PP-test).
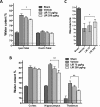
Fig 2. Effect of Liraglutide on brain water content and Neuroscore after TBI.
A: Ipsilateral total water content of the 6 mm coronal sample area (Ipsi-Total) and corresponding contralateral region (Ctrl-Total) 48 hours following controlled cortical impact (CCI). Total water content increased significantly in vehicle treated animals compared to Sham. However, water content was mitigated significantly in animals treated with Liraglutide 200 μg/kg BID. B: Subregion water content of contralateral and ipsilateral areas 48 hours after CCI. Liraglutide 200 μg/kg BID significantly mitigated cerebral water content in the ipsilateral hippocampus and thalamus after CCI. C: Composite Neuroscore test was calculated through a battery of six sub-tests 48 hours after CCI, with a score of maximum 21 points. Values in A and B are means ± SEM. *:p<0.05, **:p<0.01. Values in C are presented as median and error bars indicate the 25th and 75th percentile. Abbreviations: LIR75—Liraglutide 75 μg/kg; n = 10, LIR200—Liraglutide 200 μg/kg; n = 10, vehicle n = 10 and Sham n = 4.
Fig 3. Effect of Liraglutide on normal brain water content and Neuroscore.
Water content in the uninjured naive brain was unaffected by Liraglutide 200 μg/kg 48 hours after CCI. Values are means ± SEM. B: There was no effect of Liraglutide on Neuroscore in naive animals. Values are median with 25th and 75th percentile.
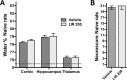
Fig 4. Paw placement and Circling test (experiment 2 and 3).
Performance of vehicle- and Liraglutide-treated rats in Circling test 2 days (A) and 7 days (B) after controlled cortical impact (CCI), and limb-placing ability for 2 days (C) and 7 days (D) after CCI. The normal score before injury is 2 for circling test and 4 for paw placement test. Values are presented as median and error bars indicate the 25th and 75th percentile, #:p<0.05.
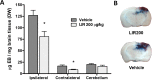
Fig 5. Evans blue exudation.
A: Analysis of Evans Blue extravasation (μg/mg of dry brain tissue) 48 hours after CCI in rats. Treatment with Liraglutide 200 μg/kg BID significantly reduced Evans Blue extravasation in both hemispheres. B: Illustration of coronal sections through the contusion center illustrates Evans blue distribution throughout the rat brain. Evans blue extravasation is particularly prominent in the pericontusional cortex, hippocampus and upper thalamus. Values are mean ± SEM. *:p<0.05.
Fig 6. Effects on lesion volume and delayed neuronal death 7 days post-injury.
A: Illustration of representative lesions by NeuN stained coronal sections from rats treated either with vehicle or Liraglutide for two days. The successive coronal sections range from +2.2 to -6.8 mm from bregma. B: Calculated cortical lesion volume (mm3). C: Calculated lesion volume in the ipsilateral thalamus (mm3). D: NeuN stained coronal section -2.8 mm from bregma illustrating the 5 regions of interest (ROI) chosen for investigation of FJC+ cells within the thalamus. Each ROI represents a counting frame at 10X. E: Demonstration of counting frame with FJC+ cells 7 days after CCI. F: Degenerating neurons shown at 20X. G: The average number of FJC+ cells for the 5 ROI’s was unaffected by Liraglutide treatment for the first 2 days post injury. *:p<0.05. Values are means ± SEM.
Similar articles
- Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice.
Li Y, Bader M, Tamargo I, Rubovitch V, Tweedie D, Pick CG, Greig NH. Li Y, et al. J Neurochem. 2015 Dec;135(6):1203-1217. doi: 10.1111/jnc.13169. Epub 2015 Jun 18. J Neurochem. 2015. PMID: 25982185 Free PMC article. - GLP-1R Agonist Liraglutide Attenuates Inflammatory Reaction and Neuronal Apoptosis and Reduces Early Brain Injury After Subarachnoid Hemorrhage in Rats.
Tu XK, Chen Q, Chen S, Huang B, Ren BG, Shi SS. Tu XK, et al. Inflammation. 2021 Feb;44(1):397-406. doi: 10.1007/s10753-020-01344-4. Epub 2020 Sep 19. Inflammation. 2021. PMID: 32951103 - Neuroprotective effects of citicoline on brain edema and blood-brain barrier breakdown after traumatic brain injury.
Başkaya MK, Doğan A, Rao AM, Dempsey RJ. Başkaya MK, et al. J Neurosurg. 2000 Mar;92(3):448-52. doi: 10.3171/jns.2000.92.3.0448. J Neurosurg. 2000. PMID: 10701532 - Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: a review.
Stutzmann JM, Mary V, Wahl F, Grosjean-Piot O, Uzan A, Pratt J. Stutzmann JM, et al. CNS Drug Rev. 2002 Spring;8(1):1-30. doi: 10.1111/j.1527-3458.2002.tb00213.x. CNS Drug Rev. 2002. PMID: 12070524 Free PMC article. Review. - The blood-brain barrier as a target in traumatic brain injury treatment.
Thal SC, Neuhaus W. Thal SC, et al. Arch Med Res. 2014 Nov;45(8):698-710. doi: 10.1016/j.arcmed.2014.11.006. Epub 2014 Nov 20. Arch Med Res. 2014. PMID: 25446615 Review.
Cited by
- Technique of ICP Monitored Stepwise Intracranial Decompression Effectively Reduces Postoperative Complications of Severe Bifrontal Contusion.
Sun G, Shi L, Pan T, Li X, Zhang S. Sun G, et al. Front Neurol. 2016 Apr 11;7:56. doi: 10.3389/fneur.2016.00056. eCollection 2016. Front Neurol. 2016. PMID: 27148158 Free PMC article. - Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice.
Li Y, Bader M, Tamargo I, Rubovitch V, Tweedie D, Pick CG, Greig NH. Li Y, et al. J Neurochem. 2015 Dec;135(6):1203-1217. doi: 10.1111/jnc.13169. Epub 2015 Jun 18. J Neurochem. 2015. PMID: 25982185 Free PMC article. - Elevated Intracranial Pressure in Cryptococcal Meningoencephalitis: Examining Old, New, and Promising Drug Therapies.
Alanazi AH, Adil MS, Lin X, Chastain DB, Henao-Martínez AF, Franco-Paredes C, Somanath PR. Alanazi AH, et al. Pathogens. 2022 Jul 10;11(7):783. doi: 10.3390/pathogens11070783. Pathogens. 2022. PMID: 35890028 Free PMC article. Review. - Activation of microglial GLP-1R in the trigeminal nucleus caudalis suppresses central sensitization of chronic migraine after recurrent nitroglycerin stimulation.
Jing F, Zou Q, Wang Y, Cai Z, Tang Y. Jing F, et al. J Headache Pain. 2021 Jul 29;22(1):86. doi: 10.1186/s10194-021-01302-x. J Headache Pain. 2021. PMID: 34325647 Free PMC article. - Glucose-Dependent Insulinotropic Polypeptide Ameliorates Mild Traumatic Brain Injury-Induced Cognitive and Sensorimotor Deficits and Neuroinflammation in Rats.
Yu YW, Hsieh TH, Chen KY, Wu JC, Hoffer BJ, Greig NH, Li Y, Lai JH, Chang CF, Lin JW, Chen YH, Yang LY, Chiang YH. Yu YW, et al. J Neurotrauma. 2016 Nov 15;33(22):2044-2054. doi: 10.1089/neu.2015.4229. Epub 2016 May 9. J Neurotrauma. 2016. PMID: 26972789 Free PMC article.
References
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129: 1021–1029. - PubMed
MeSH terms
Substances
Grants and funding
The authors received no specific funding for this work.
LinkOut - more resources
Full Text Sources
Other Literature Sources