Single cell transcriptome amplification with MALBAC - PubMed (original) (raw)
Single cell transcriptome amplification with MALBAC
Alec R Chapman et al. PLoS One. 2015.
Abstract
Recently, Multiple Annealing and Looping-Based Amplification Cycles (MALBAC) has been developed for whole genome amplification of an individual cell, relying on quasilinear instead of exponential amplification to achieve high coverage. Here we adapt MALBAC for single-cell transcriptome amplification, which gives consistently high detection efficiency, accuracy and reproducibility. With this newly developed technique, we successfully amplified and sequenced single cells from 3 germ layers from mouse embryos in the early gastrulation stage, and examined the epithelial-mesenchymal transition (EMT) program among cells in the mesoderm layer on a single-cell level.
Conflict of interest statement
Competing Interests: The authors of this manuscript have the following competing interests: SL and XSX are cofounders of Yikon Genomics, a single cell genomics start-up. This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures
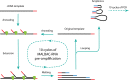
Fig 1. Single-cell MALBAC-RNA amplification diagram.
After reverse transcription, primers with 7 random nucleotides at the 3’ end are annealed to the cDNA template at 4°C, then extended by DNA polymerase with strand displacement activity as temperature is increased. Amplicons are then melted off the original template after DNA extension, and looped at 58°C to protect themselves from being further amplified thanks to their 5’ ends being complementary to their 3’ ends. This MALBAC-RNA step includes a total of 10 cycles of quasilinear amplification, followed by another 19 cycles of PCR.
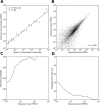
Fig 2. Technical reproducibility of MALBAC-RNA amplification.
(A) Mean expression level measured in across two technical replicates and nine SW480 single cells for synthetic spike-ins of a particular concentration. Error bars represent standard errors. (B) Scatter plot of two technical replicates exhibits a high correlation coefficient (R = 0.995). To prepare technical replicates, single-cell amount of RNA was aliquoted from 100 cells after cell membrane lysis and they should only differ by Poisson fluctuations in molecular counts. (C) Probability of detecting a transcript in one technical replicate as a function of its expression level in the other replicate. (C) Probability that the expression level of a transcript in one replicate will differ by at least 10-fold from the measurement in the other replicate.
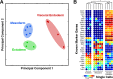
Fig 3. Gene expression profiles of 7.0dpc mouse embryo stem cells from 3 different germ layers.
MALBAC-RNA distinguishes single cells from different germ layers of a post-implantation mouse embryo (7.0dpc). A total of 12 single cells were isolated from a 7.0dpc mouse embryo, among which 3 were from the ectoderm, 5 from the mesoderm, and 4 from the visceral endoderm. (A) Principle component analysis of transcriptomes clearly separates the 12 single cells into three clusters, each representing one germ layer. (B) Top: Hierarchical clustering of transcriptomes classifies the 12 single cells into three non-overlapping sub-trees representing the three germ layers. Bottom: Known marker genes of the three germ layers exhibit strong layer-specific patterns of expression, although some show significant cell-to-cell variation within a layer. Principle component analysis and hierarchical clustering were based on the ranking of each gene’s FPKM among all cells.
Fig 4. Gene expression heat map of EMT-related genes.
Genes related to epithelial-mesenchymal transition (EMT) are differentially expressed across the three germ layers. Among them, FGF10 and Snai1 are significantly overexpressed in the mesoderm, whereas E-cadherin and Sox3 are depleted. At the same time, Eomes and Mesp1 are highly expressed in the mesoderm, although Mesp2 is not significantly expressed. Other EMT-related genes, including CDH2, Wnt5a, Wnt3, Hmga2, Smad1, and Fgf10, are also enriched in the mesoderm, which confirms the cellular transitions during gastrulation.
Similar articles
- [Comparison of different single cell whole genome amplification methods and MALBAC applications in assisted reproduction].
Yao YX, La YF, Di R, Liu QY, Hu WP, Wang XY, Chu MX. Yao YX, et al. Yi Chuan. 2018 Aug 16;40(8):620-631. doi: 10.16288/j.yczz.18-091. Yi Chuan. 2018. PMID: 30117418 Review. Chinese. - Comparison of multiple displacement amplification (MDA) and multiple annealing and looping-based amplification cycles (MALBAC) in single-cell sequencing.
Chen M, Song P, Zou D, Hu X, Zhao S, Gao S, Ling F. Chen M, et al. PLoS One. 2014 Dec 8;9(12):e114520. doi: 10.1371/journal.pone.0114520. eCollection 2014. PLoS One. 2014. PMID: 25485707 Free PMC article. - Validation of multiple annealing and looping-based amplification cycle sequencing for 24-chromosome aneuploidy screening of cleavage-stage embryos.
Huang J, Yan L, Fan W, Zhao N, Zhang Y, Tang F, Xie XS, Qiao J. Huang J, et al. Fertil Steril. 2014 Dec;102(6):1685-91. doi: 10.1016/j.fertnstert.2014.08.015. Epub 2014 Sep 17. Fertil Steril. 2014. PMID: 25241375 - Single-Cell Whole-Genome Amplification and Sequencing: Methodology and Applications.
Huang L, Ma F, Chapman A, Lu S, Xie XS. Huang L, et al. Annu Rev Genomics Hum Genet. 2015;16:79-102. doi: 10.1146/annurev-genom-090413-025352. Epub 2015 Jun 11. Annu Rev Genomics Hum Genet. 2015. PMID: 26077818 Review.
Cited by
- Single-Circulating Tumor Cell Whole Genome Amplification to Unravel Cancer Heterogeneity and Actionable Biomarkers.
Khan T, Becker TM, Po JW, Chua W, Ma Y. Khan T, et al. Int J Mol Sci. 2022 Jul 29;23(15):8386. doi: 10.3390/ijms23158386. Int J Mol Sci. 2022. PMID: 35955517 Free PMC article. Review. - Single-cell transcriptomics in cancer: computational challenges and opportunities.
Fan J, Slowikowski K, Zhang F. Fan J, et al. Exp Mol Med. 2020 Sep;52(9):1452-1465. doi: 10.1038/s12276-020-0422-0. Epub 2020 Sep 15. Exp Mol Med. 2020. PMID: 32929226 Free PMC article. Review. - Capturing the 'ome': the expanding molecular toolbox for RNA and DNA library construction.
Boone M, De Koker A, Callewaert N. Boone M, et al. Nucleic Acids Res. 2018 Apr 6;46(6):2701-2721. doi: 10.1093/nar/gky167. Nucleic Acids Res. 2018. PMID: 29514322 Free PMC article. Review. - Microfluidics for genome-wide studies involving next generation sequencing.
Ma S, Murphy TW, Lu C. Ma S, et al. Biomicrofluidics. 2017 Mar 10;11(2):021501. doi: 10.1063/1.4978426. eCollection 2017 Mar. Biomicrofluidics. 2017. PMID: 28396707 Free PMC article. Review. - Towards Improving Embryo Prioritization: Parallel Next Generation Sequencing of DNA and RNA from a Single Trophectoderm Biopsy.
Fuchs Weizman N, Wyse BA, Antes R, Ibarrientos Z, Sangaralingam M, Motamedi G, Kuznyetsov V, Madjunkova S, Librach CL. Fuchs Weizman N, et al. Sci Rep. 2019 Feb 27;9(1):2853. doi: 10.1038/s41598-019-39111-7. Sci Rep. 2019. PMID: 30814554 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Grants and funding
- T32 GM008313/GM/NIGMS NIH HHS/United States
- 5R33CA174560/CA/NCI NIH HHS/United States
- R33 CA174560/CA/NCI NIH HHS/United States
- DP1 CA186693/CA/NCI NIH HHS/United States
- 5DP1CA186693/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources