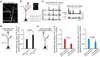Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity - PubMed (original) (raw)
Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity
Joseph Cichon et al. Nature. 2015.
Abstract
The brain has an extraordinary capacity for memory storage, but how it stores new information without disrupting previously acquired memories remains unknown. Here we show that different motor learning tasks induce dendritic Ca(2+) spikes on different apical tuft branches of individual layer V pyramidal neurons in the mouse motor cortex. These task-related, branch-specific Ca(2+) spikes cause long-lasting potentiation of postsynaptic dendritic spines active at the time of spike generation. When somatostatin-expressing interneurons are inactivated, different motor tasks frequently induce Ca(2+) spikes on the same branches. On those branches, spines potentiated during one task are depotentiated when they are active seconds before Ca(2+) spikes induced by another task. Concomitantly, increased neuronal activity and performance improvement after learning one task are disrupted when another task is learned. These findings indicate that dendritic-branch-specific generation of Ca(2+) spikes is crucial for establishing long-lasting synaptic plasticity, thereby facilitating information storage associated with different learning experiences.
Figures
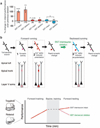
Extended Data Figure 1. The forelimb motor cortex is important for treadmill running and performance improvement
a, Representative forelimb gait traces from wild-type mice running forward on the treadmill during the first few minutes of training. Several gait patterns (drag, sweep, wobble and steady run) were observed. b, Average width between forelimbs in steady run decreased during forward treadmill running. c, Backward running elicited changes in gait patterning over two 20-min training sessions. Initially, the mice exhibited mostly steady run (34%) and drag (55%) gait patterns without sweep. With continued training, mice refined their gait from drag to steady run (75%) over 40 min. d, Average stride length in steady run increased during backward treadmill training. e, Bilateral injections of muscimol, a GABA receptor agonist, into the forelimb motor cortex acutely impaired treadmill running performance (n = 4). Muscimol injected mice displayed high percentages of untrained gait features (drag: 70% (0–5 min) and 80% (50–55 min)), whereas mice injected with saline to forelimb motor cortex did not (drag: 44% (0–5 min) and 13% (50–55 min)) (n = 5). Muscimol injections into barrel cortex did not impair treadmill running performance (drag: 34% (0–5 min) and 18% (50–55 min)) (n = 4). Data are presented as mean ± s.e.m. *P < 0.05, ***P < 0.001, paired _t_-test. Extended Data
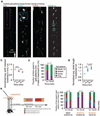
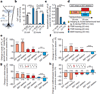
Extended Data Figure 2. Detection of motor learning-induced Ca2+ spikes by various GCaMPs in apical tuft dendrites of layer 5 neurons in the motor cortex
a, b, Coronal sections of forelimb motor cortex from mice expressing AAV-GCaMP5G (a) or GCaMP2.2c (b). Boxed regions shows the expression of GCaMPs in L5. c, Two-photon images of GCaMP2.2c-, 3- and 5G-expressing dendrites during quiet resting state and forward running. Images of baseline Ca2+ signals under quiet resting state (top) and running-induced Ca2+ spikes (bottom; yellow arrowheads) are shown. d, e, Fast-scanning of apical tuft dendrites during forward running in mice expressing GCaMP2.2c (n = 18) and GCaMP5G (n = 14). Grey traces are individual Ca2+ transients and black trace represents the average. f, Average rise and decay times of apical tuft Ca2+ spikes during running for different GCaMPs (unpaired _t_-test). g, Measurements of Ca2+ fluorescence along long dendritic segments in the plane of imaging. Both GCaMP6s (n = 10) and GCaMP2.2c (n = 7) detected comparable fluorescent signals across entire dendritic segments. h, Number of Ca2+ spikes during quiet resting, running forward, running backward, and with local application of MK801(paired _t_-test). Ca2+ spikes were detected on L5 tuft branches in an image field (160 × 80 µm) over 2.5 min in Thy-1 GCaMP2.2c transgenic mice. i, The number of dendritic Ca2+ spikes generated in early running trials was not significantly different from that in later (30 min) running trials (P = 0.89, paired _t_-test). Scale bar, 50 µm (a, b) and 15 µm (c). *P < 0.05, *** P < 0.001. Extended Data
Extended Data Figure 3. The overlap of Ca2+ activity in apical dendritic trunk, somata, and apical tuft branches of L5 pyramidal neurons in the motor cortex in response to treadmill running
a, Frequency distribution of the number of Ca2+ transients detected over 2.5 min in individual apical trunks in mice running forward and backward. b, Ca2+ imaging of layer 5 somata during forward and backward running. c, Frequency distribution of the number of Ca2+ transients in individual L5 somata detected over 2.5 min in mice running forward and backward (n = 504 cells from 10 mice). d, Layer 5 somata responded to multiple tasks when running in four directions (n = 242 cells from 4 mice). e, Summary of the overlap of Ca2+ activity in response to forward and backward running across different cortical layers in the motor cortex. f, Two-dimensional projection of six apical tuft branches from the same L5 neuron expressing GCaMP6s. Six ROIs corresponding to different branches were analysed over 2.5 min forward or backward running. Green arrowhead marks the location of the trunk (~200 µm below the pia). Note that there is little or no overlap between forward spikes and backward spikes in these branches. g, Two-dimensional two-photon image of four apical tuft branches expressing GCaMP2.2c from an individual L5 neuron. Green arrowhead marks the trunk. Four ROIs corresponding to different branches were analysed over four trials of forward and backward running. ROI 2 and 3 generated Ca2+ transients in response to backward running. h, Distribution of forward and backward running-induced Ca2+ spikes on 33 sibling branches located 0–100 µm below the pia. Data were analysed over five 30-s trials of forward and backward running. i, j, Distribution of forward and backward running-induced Ca2+ spikes on 80 sibling branches located 100–200 µm below the pia (22 cells). Approximately 43% branches at this cortical depth exhibited spikes in response to both forward and backward running. Each of these branches was connected to higher order branches that were either inactive or exhibited spikes in response to forward, backward or both running modes (dotted boxes in i). k, Percentage of local or global Ca2+ spikes observed on sibling branches located at two different depths below the pia. **P < 0.01, ***P < 0.001, unpaired _t_-test. Extended Data
Extended Data Figure 4. In vivo two-photon laser cutting of one apical tuft branch reduces calcium activity at the apical trunk
a–c, Example of an individual neuron with three apical tuft branches (at 150 µm) and the apical trunk (at 300 µm; a) showed Ca2+ spikes during forward running. Laser cutting one tuft branch (b) induced a significant reduction in average peak Δ_F/F_0 of the trunk during forward running (c). d, Summary of average peak Δ_F/F_0 of the trunk before and after parking the laser beam in a region 30 µm away from the active dendrite (control cut; n = 7, P = 0.22, paired _t_-test). There was no significant change in the activity of the trunk in this control experiment. e, Summary of average peak Δ_F/F_0 of the trunk before and after cutting an active dendrite during forward (n = 7, P < 0.001, paired _t_-test) and backward running (P = 0.25; n = 5). ***P < 0.001. As expected, cutting a dendritic branch exhibiting forward running-induced Ca2+ spikes reduced the activity of the trunk when animals ran in the forward direction (left). When the branch with forward Ca2+ spikes was cut, the average activity of the trunk was also reduced for the backward direction (right), even though the uncut branch still exhibited Ca2+ spikes in response to backward running. This is probably related to the fact that apical tufted branches possess spines that are active during both forward and backward running (data not shown). Cutting a branch eliminated the contribution of not only dendritic Ca2+ spikes but also synaptic inputs to the depolarization at the trunk. Extended Data
Extended Data Figure 5. Ca2+ spikes cause long-lasting potentiation of task-related dendritic spines
a, Frequency distribution of the number of Ca2+ transients in individual spines detected by GCaMP6s over 2.5-min running (n = 199 spines from 14 dendritic branches). b, Frequency distribution of the peak amplitude (Δ_F/F_0) of spine Ca2+ transients on apical tuft branches expressing GCaMP6s during running. c, Frequency distribution of spine Ca2+ transient duration during running. d, Frequency distribution of the number of spine Ca2+ transients detected by GCaMP2.2c over 2.5-min running. e, Frequency distribution of the peak amplitude (Δ_F/F_0) of spine Ca2+ transients induced by forward running in tuft dendrites expressing GCaMP2.2c. As expected, spine Ca2+ transients detected with GCaMP6s were significantly higher than those detected by GCaMP2.2c in terms of amplitude (>200%) and frequency (>150%). f, Task-specific activation of dendritic spines detected by GCaMP2.2c during forward and backward running (n = 98 spines from 12 dendrites). g, Two-photon images of an apical tuft dendrite expressing GCaMP2.2c. An active spine (green) denoted by yellow arrowhead and a Ca2+ spike (red) are shown. h, i, Representative two-photon images and fluorescent traces of potentiated spines on L5 apical tuft dendritic segments expressing GCaMP2.2c and 5G. Ca2+ spikes occurred during trial 5 in h and i. j, Many spines were active before, during and after the generation of Ca2+ spikes. Horizontal lines indicate the start and end of spine Ca2+ transients. Variable Ca2+ spike duration indicated by red shaded bar. k, l, Comparison of spine head and shaft fluorescence for spines active at the time of spike generation versus neighbouring spines that are not active at the time of spike generation. Δ_F/F_0 of active spine heads are significantly larger (P < 0.001; 236.5% (GCaMP6s); 319.2% (GCaMP2.2c)) than that of neighbouring inactive spines during Ca2+ spike generation. m, Percentage change in average peakΔ_F/F_0 of spine Ca2+ transients before and after spikes detected by GCaMP6s (green) and GCaMP2.2c (red). The peaks of 3–4 spine Ca2+ transients (Δ_F/F_0) were averaged before and after the spike. There was no significant difference in the degree of spine Ca2+ transient potentiation detected by GCaMP6s (green) and GcaMP2.2c (red) (P = 0.22, unpaired _t_-test). n, Percentage change in the peakΔ_F/F_0 of individual spine Ca2+ transients (no average) immediately before and after spikes detected byGCaMP6s (green) andGCaMP2.2c (red) (P = 0.16, unpaired _t_-test). o, Comparison of spine size versus the percentage change in the peak amplitude of spine Ca2+ transients at 2 min and 40 min post spike. Data are mean ± s.e.m. Scale bars, 5 µm. Extended Data
Extended Data Figure 6. CaMKII inhibitors block potentiation of task-related dendritic spines but not Ca2+ spike generation
a, There is no correlation between the percentage change in the peak amplitude of spine Ca2+ transients and the peak amplitude of dendritic Ca2+ spike during forward running in the presence of CaMKII inhibitors (KN-62: P = 0.08; KN-93: P = 0.42, Pearson’s correlation). b, Two-photon images of apical tuft dendrites expressing GCaMP2.2c during quiet resting state and forward running in the presence of ACSF, KN-93 and KN-62. c, d, Local application of CaMKII inhibitors, KN-93 and KN-62, to layer 1 did not induce significant changes in Ca2+ spike frequency or peak amplitude as compared to ACSF controls (_P_> 0.05, unpaired _t_-test). Scale bar, 10 µm. Data are mean ± s.e.m. Extended Data
Extended Data Figure 7. Spine activity relative to spike generation and task switching
a, b, In mice running forward, most spines (54%, red) exhibited activities at the time of the spike generation (near t = 0). Only a small fraction of spines were active < 5 s before Ca2+ spike generation (17%, green). Thus, most spines active during forward running coincide with the spike generation. Of the spines that were active asynchronously relative to Ca2+ spikes, the average time interval between the two events was 12.2 ± 1.6 s. **c**, **d**, Changes in the peak amplitude of Ca2+ transients in forward running-activated spines versus the time interval between spine activity and the onset of task switching from forward to backward running. **e**, Spines active < 5 s or >5 s before task switching (no backward spike) show no significant reduction in the peak amplitude of Ca2+ transients afterwards (< 5 s: _P_ = 0.31; _n_ = 9; >5 s: P = 0.84; n = 22; unpaired _t_-test). f, There are no significant changes in the peak amplitude of spine Ca2+ transients (no backward spike) during the first and second half of a 30-s forward running trial, either before or after the task switching (paired _t_-test). Thus, spine transients do not gradually decrease during the initial forward running and recover to their highest values after the second forward running session is switched on. This suggests that the depotentation of spines active within 5 s before spikes in Fig. 3 is related to their interactions with local Ca2+ spikes. Extended Data
Extended Data Figure 8. The effect of inactivating SST neurons on Ca2+ spikes and potentiation of task-related dendritic spines
a, Experimental design for perturbing branch-specific Ca2+ spikes by applying bicuculline (BIC) to L1. Local bicuculline administration did not have a significant effect (_P_>0.05, paired _t_-test) on the number of Ca2+ spikes generated during forward running as compared to control mice. Before bicuculline: 60.2 ± 10.2 Ca2+ spikes over 2.5 min; after bicuculline application: 77.4 ± 13.7 Ca2+ spikes over 2.5 min. More than 200 Ca2+ spikes were measured under each condition from five mice. b, Local bicuculline administration increased the percentage of apical tuft branches exhibiting Ca2+ spikes during both forward and backward running (n = 5 mice, P = 0.007, paired _t_-test). c, Coronal sections of the motor cortex from control or SST-deleted mice stained for parvalbumin (PV) 2 days after diphtheria toxin administration. No significant effect on the number of parvalbumin cells was observed (P = 0.30, unpaired _t_-test). d, Coronal sections of motor cortex from control or SST-deleted mice stained for microglia (Iba1) 2 days after diphtheria toxin administration. No significant effect on the number of microglia was observed (P = 0.50, unpaired _t_-test). e, Two-photon images and quantification of SST cells expressing GCaMP6 and hM4Di-mCherry. f, Training protocol (left) to test the effect of inactivating SST cells after CNO treatment on branch-specific Ca2+ spike generation. Two-photon images (right) of apical tuft dendrites expressing GCaMP2.2c during forward and backward running before and after SST neuron silencing. Note that tuft dendrites after CNO exhibited Ca2+ spikes during both forward and backward running. g, The average peak amplitude of Ca2+ spikes during resting and running in SST-deleted and control mice expressing GCaMP2.2c. The peak Δ_F/F_0 of Ca2+ spikes during quiet resting and forward running in SST-deleted mice was significantly higher than in control mice (P < 0.001, unpaired _t_-test). h, In SST-deleted mice, spines active at the time of spike generation show enhanced Ca2+ signals after spike generation (n = 49. P < 0.001, paired _t_-test)), whereas spines not active at the spike generation (n = 27 spines) showed no significant increase in spine Ca2+ signals. In SST-deleted mice, the ratio of Ca2+ fluorescence intensity between spine heads and neighbouring shafts was 2.03 ± 0.23, significantly higher than that for neighbouring inactive spines (0.61 ± 0.03). Data are mean ± s.e.m. **P < 0.01, ***P < 0.001. Extended Data
Extended Data Figure 9. CaMKII inhibitors block training-related potentiation of Ca2+ transients in L5 apical trunk nexus
a, Texas Red dye injection in L5 or L1. b, Fluorescence signals spread to a region with a diameter~237 ± 19 µmin L5 and 188 ± 10 µmin L1 respectively. There is no overlap between the sites of injection in L5 and L1. c, AverageΔ_F/F_0 of L5 soma detected by GCaMP6s over 2.5 min running under various conditions (no injection, L1 ACSF injection, L1 TTX injection) (P < 0.001, unpaired _t_-test). d, e, In control mice, local application of CaMKII inhibitors (KN-62 and KN-93) blocked the increase in Ca2+ transients at the apical trunk nexus after an initial and second training session of forward running.
Extended Data Figure 10. Deletion of somatostatin-expressing interneuron impairs performance improvement after motor learning
a, Rotarod performance in control and SST-deleted mice subjected to forward–forward running or forward–backward running. When forward running on the accelerated rotarod was followed by backward running, SST-deleted mice displayed a reduction in their performance (the average speed animals achieved) as compared to control mice when tested again in forward running (P < 0.05, unpaired _t_-test). Data are mean ± s.e.m. b, A model showing the importance of BSDCS for inducing synaptic changes that affect L5 neuronal output duringmotor skill learning. Dendritic spines active (1, pink) at the time of spike generation (2) show enhanced Ca2+ activity and changes in synaptic strength (red) following the Ca2+ spike. Ca2+ spike-induced potentiation of synapses contributes to persistent synaptic changes, potentiated Ca2+ activity at the apical trunk and L5 soma, as well as improvements in performance over training sessions. In control mice, different motor tasks (that is, forward, backward running) induce Ca2+ spikes on different tuft branches of L5 neurons (not shown). Inactivation of SST interneurons results in individual dendritic branches generating Ca2+ spikes in response to both tasks (3). Loss of spatial segregation of Ca2+ spikes results in the depotentiation of synaptic changes-induced by previous learning in tuft dendritic branches and reduced Ca2+ activity in the apical trunk nexus and L5 soma when a different task is learned. SST interneuron inactivation induces a state of interference that impairs motor performance when several tasks are learned.
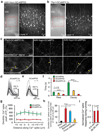
Figure 1. Motor learning induces branch-specific Ca2+ spikes in apical tuft dendrites of L5 pyramidal neurons in motor cortex
a, Schematic of two-photon Ca2+ imaging at different cortical depths during treadmill training. b, Changes of forelimb gait patterns between early and late trials. c, Average stride length in steady run increased after training (n = 9 mice, paired _t_-test). d, Images of tuft dendrites expressing GCaMP6s during resting and FWR. Running-induced Ca2+ transients were visible over long dendritic segments (yellow arrowheads). e, Fast-scanning of individual Ca2+ transients (grey traces, n = 24) during FWR. Red trace represents the average. f, The number of Ca2+ transients increased >7-fold during FWR or BWR relative to resting (P = 2.6 × 10−7, paired _t_-test). g, Distribution of Ca2+ spike frequency on individual branches during FWR (n = 321), BWR (n = 261) and FWR with local MK801 (n = 34) over 2.5 min. h, Distribution of peak Ca2+ spike amplitudes detected with GCaMP6s and GCaMP2.2c during FWR with or without MK801 (n = 141, 213 and 31, respectively; P < 0.0001, Mann–Whitney test). Δ_F/F_0 values of Ca2+ spikes detected with GCaMP6s were ~8.5 times larger than with GCaMP2.2c. i, 95% of tuft branches that spiked were activated by only one task (n = 616 for two tasks; n = 450 for four tasks). j, 53% of apical trunks exhibited Ca2+ transients in response to several tasks (n = 257). k, Sibling branches exhibited non-overlapping FWR- and BWR-induced Ca2+ spikes. l, Two-dimensional projection of multiple sibling branches. Green arrowhead marks the trunk. Six regions of interests (ROIs) corresponding to different branches were analysed over five trials of FWR and BWR. m, The percentage of sibling branches with overlapping FWR/BWR-induced Ca2+ spikes at two cortical depths below the pia. Data are mean ± s.e.m. ***P < 0.001. See Methods for statistical details.
Figure 2. Ca2+ spikes cause persistent potentiation of task-related dendritic spines
a, b, Time-lapse images and fluorescent traces of a representative apical tuft branch and spines expressing GCaMP6s. Running-induced Ca2+ transients in spines (arrowheads point to peak Ca2+ signals for spine 1) and a Ca2+ spike (marked by red bar). c, Spines active during spikes showed a significant increase in the peak amplitude of Ca2+ transients after Ca2+ spikes during FWR (n = 80) and BWR (n = 18), whereas spines not active during spikes (P = 0.76; n = 22 spines), or active spines with no encounter of spikes (P = 0.15; n = 35), showed no increase. Local application of MK801 (n = 31), KN-93 (n = 20) and KN-62 (n = 18), but not artificial cerebrospinal fluid (ACSF), blocked potentiation of spine Ca2+ transients (_P_> 0.05). d, e, Change in the peak amplitude of spine Ca2+ transients correlates with the peak amplitude of Ca2+ spike (d) and the fluorescence ratio between spine head and dendritic shaft (e) for spines active at the time of spike generation during FWR (P < 0.001 for GCaMP6s and GCaMP2.2c, Pearson correlation). f, Ca2+ spikes induced persistent potentiation of spine Ca2+ transients over 40 min (n = 28). g, h, Spines undergoing Ca2+ spikes-induced Ca2+ potentiation, not neighbouring inactive spines, also showed an increase in spine head size over 40 min (n = 8) but not over 2 min (n = 11). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, *** P < 0.001, paired _t_-test. See Methods for statistical details.
Figure 3. Ca2+ spikes depotentiate spines active seconds before spike generation
a, A task-switching protocol for inducing spine activity and Ca2+ spike asynchronously. b, Changes in the peak amplitude of Ca2+ transients in FWR-activated spines versus the time interval between spine activity and a BWR-induced Ca2+ spike. c, Changes in the peak amplitude of spine Ca2+ transients under various conditions. Spines active within 5 s before the spike generation show a significant reduction in the peak amplitude of Ca2+ transients when measured again during FWR training (n = 18 spines), whereas spines active >5 s before the spike (n = 31 spines) or active spines without encountering spikes (n = 31 spines) showed no reduction. Local application of MK801 blocked the reduction. d, No correlation between the percentage change in the peak amplitude of spine Ca2+ transients and Ca2+ spike amplitude (Pearson correlation). Data are mean ± s.e.m. ***P < 0.001, paired _t_-test and Wilcoxon matched-pairs signed rank test. See Methods for statistical details.
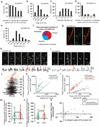
Figure 4. Disrupting cortical inhibition alters branch-specific Ca2+ spikes and synaptic potentiation
a, Two-photon images of SST-interneurons labelled with green fluorescent protein (GFP) in motor cortex before and 2 days after diphtheria toxin (DT) treatment. Loss of SST interneurons occurred within 2 days after diphtheria toxin treatment. b, Somatic Ca2+ activity was reduced after CNO in SST interneurons expressing hM4Di-mCherry/GCaMP6s (inset). c, Two-photon images of tuft dendrites exhibiting spikes during FWR and BWR after SST deletion. d, SST deletion or silencing significantly increased the percentage of tuft dendrites exhibiting Ca2+ spikes during both FWR and BWR (SST/DTR: P = 0.0007; SST/hM4Di: P = 0.002). e, Inactivating SST interneurons did not affect the number of Ca2+ spikes generated during FWR as compared to control mice (_P_> 0.05). More than 200 Ca2+ spikes were measured per condition. del., deletion. f, Training protocol to test the effect of disrupting branch-specific Ca2+ spikes on potentiated spine Ca2+ transients in control and SST-deleted mice. g, Pie charts showing 44% and 71% of FWR-potentiated spines were depotentiated during BWR in control and SST-deleted mice, respectively. h, Spines potentiated during FWR were depotentiated when active <5 s before BWR-induced Ca2+ spikes in SST-deleted mice. i, Summary of the effect of FWR or BWR on previously potentiated spines in control and SST-deleted mice. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, paired _t_-test (a, d, i) and Wilcoxon matched-pairs signed rank test (b, i). Statistical details in Methods.
Figure 5. Deletion of SST-interneurons impairs soma activities of L5 pyramidal neurons and performance improvement after learning
a–c, TTX applied to L1, but not L5, inhibited Ca2+ spike generation in the apical tuft and trunk. TTX to L1 also reduced Ca2+ transients in L5 somata during FWR. d, Experimental design to assess the effect of disrupting branch-specific Ca2+ spike on activities of apical trunks and somata, as well as behavioural performance in SST-deleted mice. e, f, In control and SST-deleted mice, Ca2+ activity at the trunk nexus (control: n = 64; deleted: n = 61) and L5 soma (control: n = 113; deleted: n = 74) increased after an initial and second sessions ofFWR.BWR after the initial FWR significantly reduced Ca2+ activity at trunk (control: n = 107; deleted: n = 77) and L5 somata (control: n = 63; deleted: n = 67) in SST-deleted mice when compared to control mice. g, h, When FWR was followed by BWR, SST-deleted mice, but not control mice, displayed a reduction of the stride length and an increase in the width between forelimbs when tested in FWR. Number of mice per group is indicated. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, *** P < 0.001, Mann–Whitney U test (b–f) and paired _t_-test (g, h). See Methods for statistical details. Extended Data
Similar articles
- Dendritic calcium mechanisms and long-term potentiation in cortical inhibitory interneurons.
Topolnik L. Topolnik L. Eur J Neurosci. 2012 Feb;35(4):496-506. doi: 10.1111/j.1460-9568.2011.07988.x. Epub 2012 Feb 6. Eur J Neurosci. 2012. PMID: 22304664 Review. - Direction of action potential propagation influences calcium increases in distal dendrites of the cricket giant interneurons.
Ogawa H, Baba Y, Oka K. Ogawa H, et al. J Neurobiol. 2002 Oct;53(1):44-56. doi: 10.1002/neu.10105. J Neurobiol. 2002. PMID: 12360582 - Dendritic calcium spikes induce bi-directional synaptic plasticity in the lateral amygdala.
Humeau Y, Lüthi A. Humeau Y, et al. Neuropharmacology. 2007 Jan;52(1):234-43. doi: 10.1016/j.neuropharm.2006.07.010. Epub 2006 Aug 4. Neuropharmacology. 2007. PMID: 16890250 - Rapid formation and selective stabilization of synapses for enduring motor memories.
Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Xu T, et al. Nature. 2009 Dec 17;462(7275):915-9. doi: 10.1038/nature08389. Epub 2009 Nov 29. Nature. 2009. PMID: 19946267 Free PMC article. - Cooperative LTP can map memory sequences on dendritic branches.
Mehta MR. Mehta MR. Trends Neurosci. 2004 Feb;27(2):69-72. doi: 10.1016/j.tins.2003.12.004. Trends Neurosci. 2004. PMID: 15106650 Review.
Cited by
- A dendritic disinhibitory circuit mechanism for pathway-specific gating.
Yang GR, Murray JD, Wang XJ. Yang GR, et al. Nat Commun. 2016 Sep 20;7:12815. doi: 10.1038/ncomms12815. Nat Commun. 2016. PMID: 27649374 Free PMC article. - Fast 3D Imaging of Spine, Dendritic, and Neuronal Assemblies in Behaving Animals.
Szalay G, Judák L, Katona G, Ócsai K, Juhász G, Veress M, Szadai Z, Fehér A, Tompa T, Chiovini B, Maák P, Rózsa B. Szalay G, et al. Neuron. 2016 Nov 23;92(4):723-738. doi: 10.1016/j.neuron.2016.10.002. Epub 2016 Oct 20. Neuron. 2016. PMID: 27773582 Free PMC article. - Two-photon calcium imaging of neuronal activity.
Grienberger C, Giovannucci A, Zeiger W, Portera-Cailliau C. Grienberger C, et al. Nat Rev Methods Primers. 2022;2(1):67. doi: 10.1038/s43586-022-00147-1. Epub 2022 Sep 1. Nat Rev Methods Primers. 2022. PMID: 38124998 Free PMC article. - Enhanced Dendritic Compartmentalization in Human Cortical Neurons.
Beaulieu-Laroche L, Toloza EHS, van der Goes MS, Lafourcade M, Barnagian D, Williams ZM, Eskandar EN, Frosch MP, Cash SS, Harnett MT. Beaulieu-Laroche L, et al. Cell. 2018 Oct 18;175(3):643-651.e14. doi: 10.1016/j.cell.2018.08.045. Cell. 2018. PMID: 30340039 Free PMC article. - Cell-type-specific responses to associative learning in the primary motor cortex.
Lee C, Harkin EF, Yin X, Naud R, Chen S. Lee C, et al. Elife. 2022 Feb 3;11:e72549. doi: 10.7554/eLife.72549. Elife. 2022. PMID: 35113017 Free PMC article.
References
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. - PubMed
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nature. Rev. Neurosci. 2008;9:65–75. - PubMed
- Abraham WC, Robins A. Memory retention-the synaptic stability versus plasticity dilemma. Trends Neurosci. 2005;28:73–78. - PubMed
- Jaffe DB, et al. The spread ofNa1 spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature. 1992;357:244–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous