A three-pool model dissecting readily releasable pool replenishment at the calyx of held - PubMed (original) (raw)
A three-pool model dissecting readily releasable pool replenishment at the calyx of held
Jun Guo et al. Sci Rep. 2015.
Erratum in
- Erratum: A Three-Pool Model Dissecting Readily Releasable Pool Replenishment at the Calyx of Held.
Guo J, Ge JL, Hao M, Sun ZC, Wu XS, Zhu JB, Wang W, Yao PT, Lin W, Xue L. Guo J, et al. Sci Rep. 2016 Aug 25;6:31277. doi: 10.1038/srep31277. Sci Rep. 2016. PMID: 27560804 Free PMC article. No abstract available.
Abstract
Although vesicle replenishment is critical in maintaining exo-endocytosis recycling, the underlying mechanisms are not well understood. Previous studies have shown that both rapid and slow endocytosis recycle into a very large recycling pool instead of within the readily releasable pool (RRP), and the time course of RRP replenishment is slowed down by more intense stimulation. This finding contradicts the calcium/calmodulin-dependence of RRP replenishment. Here we address this issue and report a three-pool model for RRP replenishment at a central synapse. Both rapid and slow endocytosis provide vesicles to a large reserve pool (RP) ~42.3 times the RRP size. When moving from the RP to the RRP, vesicles entered an intermediate pool (IP) ~2.7 times the RRP size with slow RP-IP kinetics and fast IP-RRP kinetics, which was responsible for the well-established slow and rapid components of RRP replenishment. Depletion of the IP caused the slower RRP replenishment observed after intense stimulation. These results establish, for the first time, a realistic cycling model with all parameters measured, revealing the contribution of each cycling step in synaptic transmission. The results call for modification of the current view of the vesicle recycling steps and their roles.
Figures
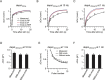
Figure 1. More intense stimulation slows the RRP replenishment.
(A) Left: Sampled presynaptic calcium current (ICa, upper) and membrane capacitance (Cm, lower) induced by a 20 ms depolarisation followed by a conditioning pulse of 20 ms depolarization with a 0.5 s interval. Right: Similar to Left, except that the stimulus interval is 20 s. (B) Upper: The protocol used to measure the RRP replenishment after a 20 ms depolarisation pulse. Lower: Cm induced by a 20 ms depolarisation applied at various intervals after the conditioning stimulus (n = 8). Data were normalised to the Cm induced by the conditioning pulse, and fit with a bi-exponential function (solid line) where A1 = 0.71, τ1 = 0.26 s, A2 = 0.29, τ2 = 9.5 s. (C) Similar to A, except that the conditioning stimulus was 10 pulses of 20 ms depolarisation at 10 Hz. (D) Similar to B, except that the conditioning stimulus was 10 pulses of 20 ms depolarisation at 10 Hz (n = 11). Data were normalised to the Cm induced by a 20 ms depolarisation applied at >30 s after the conditioning stimulus, and fit with a bi-exponential function (solid line) where A1 = 0.33, τ1 = 0.38 s, A2 = 0.67, τ2 = 7.8 s. The fitting curve of single pulse was also plotted for comparison (dotted line). (E) Similar to A, except that the conditioning stimulus was 10 pulses of 20 ms depolarisation at 1 Hz. (F) Similar to D, except that the conditioning stimulus was 10 pulses of 20 ms depolarisation at 1 Hz (n = 6). Data were fit with a bi-exponential function where A1 = 0.29, τ1 = 0.25 s, A2 = 0.71, τ2 = 7.9 s. (G) The plot of the normalised RRP replenishmentrapid amplitude versus calcium influx (QICa) in a 20 ms depolarisation pulse and 10 pulses of 20 ms depolarisation at 1–10 Hz (QICa: 38.9 ± 2.6 pC, n = 8, single pulse; 312 ± 34 pC, n = 11, 10 pulses at 10 Hz; 323 ± 15 pC, n = 6, 10 pulses at 1 Hz).
Figure 2. A three-pool model underlies rapid and slow RRP replenishment.
(A) The model-predicted RRP replenishment curves with endocytosed vesicles recycling to RP (black), IP (red) and RRP (blue) after a 20 ms depolarisation pulse. The measured data are also plotted for comparison (circle, same as Fig. 1B). The legend also applies to B and C. (B) Similar to A, but with a conditioning stimulus of 10 pulses of 20 ms depolarisation at 10 Hz. (C) Similar to A and B, but with a conditioning stimulus of 10 pulses of 20 ms depolarisation at 1 Hz. (D) The total measured and predicted ΔCm with and without endocytosis induced by 10 pulses of 20 ms depolarisation at 10 Hz (n = 11). (E) The model-predicted (with endocytosis: black curve, without endocytosis: dotted curve) and the measured (circle) ΔCm induced by each depolarising pulse (20 ms depolarisation) during a 10-pulse train at 1 Hz. (F) Similar to D, except that the conditioning stimulus was 10 pulses of 20 ms depolarisation at 1 Hz (n = 6).
Figure 3. Rapid and slow endocytosis do not recycle vesicles in a small recycling pool.
(A) Left: Sampled presynaptic calcium current (ICa, upper) and membrane capacitance (Cm, lower) induced by a 20 ms depolarisation applied at 0.5 s after a conditioning pulse of 20 ms depolarization with 0.3 mM GTPγS in place of GTP in the pipette solution. Right: Similar to Left, except that the stimulus interval is 20 s. (B) The model-predicted RRP replenishment curves with (scheme 1, black) and without endocytosis (scheme 2, red) after a 20 ms depolarisation pulse. Data measured with 0.3 mM GTPγS in the pipette solution are also plotted for comparison (circle). (C) Similar to A, but with a conditioning stimulus of 10 pulses of 20 ms depolarisation at 10 Hz. (D) Similar to B, except that the conditioning stimulus was 10 pulses of 20 ms depolarisation at 10 Hz.
Figure 4. Impact of endocytosis during high frequency stimulation.
(A) Sampled trace of EPSC recordings during a 50 Hz action potential train. Inset shows the large initial EPSC and the small stabilised EPSC for comparison. (B) The model-predicted (curves) and the measured (n = 4, circle) amplitudes of the EPSCs during a 50 Hz action potential train. Predicted traces for different depleting percentages after a single action potential are shown in different colours (yellow: 6%, red: 9%, green: 12%). Data were normalised to the first response. Each circle represents the mean amplitude from four synapses (for clarity, s.e.m. is not included). Experimental data were collected from horizontal brain slices, where a bipolar electrode was positioned in the midline of the trapezoid body to induce presynaptic action potentials and thus EPSCs. The model included endocytosis (scheme 1). (C) The model-predicted EPSC amplitude during action potential stimulation at 50 Hz with (scheme 1, colours meanings are the same as B) and without (scheme 2, black) endocytosis. Left and right panels show the same data at different scales.
Similar articles
- Ca-dependence of synaptic vesicle exocytosis and endocytosis at the hippocampal mossy fibre terminal.
Miyano R, Miki T, Sakaba T. Miyano R, et al. J Physiol. 2019 Aug;597(16):4373-4386. doi: 10.1113/JP278040. Epub 2019 Jul 25. J Physiol. 2019. PMID: 31294821 - Rapid endocytosis does not recycle vesicles within the readily releasable pool.
Wu XS, Wu LG. Wu XS, et al. J Neurosci. 2009 Sep 2;29(35):11038-42. doi: 10.1523/JNEUROSCI.2367-09.2009. J Neurosci. 2009. PMID: 19726662 Free PMC article. - Regulation of synaptic vesicle recycling by calcineurin in different vesicle pools.
Kumashiro S, Lu YF, Tomizawa K, Matsushita M, Wei FY, Matsui H. Kumashiro S, et al. Neurosci Res. 2005 Apr;51(4):435-43. doi: 10.1016/j.neures.2004.12.018. Epub 2005 Jan 24. Neurosci Res. 2005. PMID: 15740806 - Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: lessons from the Drosophila neuromuscular junction.
Kuromi H, Kidokoro Y. Kuromi H, et al. Neuroscientist. 2005 Apr;11(2):138-47. doi: 10.1177/1073858404271679. Neuroscientist. 2005. PMID: 15746382 Review. - How the stimulus defines the dynamics of vesicle pool recruitment, fusion mode, and vesicle recycling in neuroendocrine cells.
Cárdenas AM, Marengo FD. Cárdenas AM, et al. J Neurochem. 2016 Jun;137(6):867-79. doi: 10.1111/jnc.13565. Epub 2016 May 2. J Neurochem. 2016. PMID: 26849771 Review.
Cited by
- The readily releasable pool of synaptic vesicles.
Kaeser PS, Regehr WG. Kaeser PS, et al. Curr Opin Neurobiol. 2017 Apr;43:63-70. doi: 10.1016/j.conb.2016.12.012. Epub 2017 Jan 16. Curr Opin Neurobiol. 2017. PMID: 28103533 Free PMC article. Review. - Action potential-coupled Rho GTPase signaling drives presynaptic plasticity.
O'Neil SD, Rácz B, Brown WE, Gao Y, Soderblom EJ, Yasuda R, Soderling SH. O'Neil SD, et al. Elife. 2021 Jul 16;10:e63756. doi: 10.7554/eLife.63756. Elife. 2021. PMID: 34269176 Free PMC article. - The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms.
Chanaday NL, Cousin MA, Milosevic I, Watanabe S, Morgan JR. Chanaday NL, et al. J Neurosci. 2019 Oct 16;39(42):8209-8216. doi: 10.1523/JNEUROSCI.1158-19.2019. J Neurosci. 2019. PMID: 31619489 Free PMC article. Review. - Secreted C-type lectin regulation of neuromuscular junction synaptic vesicle dynamics modulates coordinated movement.
Bhimreddy M, Rushton E, Kopke DL, Broadie K. Bhimreddy M, et al. J Cell Sci. 2021 May 1;134(9):jcs257592. doi: 10.1242/jcs.257592. Epub 2021 May 11. J Cell Sci. 2021. PMID: 33973638 Free PMC article. - Increased presynaptic excitability in a migraine with aura mutation.
Suryavanshi P, Sawant-Pokam P, Clair S, Brennan KC. Suryavanshi P, et al. Brain. 2024 Feb 1;147(2):680-697. doi: 10.1093/brain/awad326. Brain. 2024. PMID: 37831655 Free PMC article.
References
- Abbott L. F. & Regehr W. G. Synaptic computation. Nature 431, 796–803 (2004). - PubMed
- Delgado R., Maureira C., Oliva C., Kidokoro Y. & Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron 28, 941–953 (2000). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous