Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection - PubMed (original) (raw)
doi: 10.1186/s40168-015-0070-0. eCollection 2015.
Antonio González 2, Yoshiki Vázquez-Baeza 3, Sophie Weiss 4, Gregory Humphry 5, Donna Berg-Lyons 5, Dan Knights 6, Tatsuya Unno 7, Aleh Bobr 8, Johnthomas Kang 9, Alexander Khoruts 9, Rob Knight 10, Michael J Sadowsky 1
Affiliations
- PMID: 25825673
- PMCID: PMC4378022
- DOI: 10.1186/s40168-015-0070-0
Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection
Alexa Weingarden et al. Microbiome. 2015.
Abstract
Background: Fecal microbiota transplantation (FMT) is an effective treatment for recurrent Clostridium difficile infection (CDI) that often fails standard antibiotic therapy. Despite its widespread recent use, however, little is known about the stability of the fecal microbiota following FMT.
Results: Here we report on short- and long-term changes and provide kinetic visualization of fecal microbiota composition in patients with multiply recurrent CDI that were refractory to antibiotic therapy and treated using FMT. Fecal samples were collected from four patients before and up to 151 days after FMT, with daily collections until 28 days and weekly collections until 84 days post-FMT. The composition of fecal bacteria was characterized using high throughput 16S rRNA gene sequence analysis, compared to microbiota across body sites in the Human Microbiome Project (HMP) database, and visualized in a movie-like, kinetic format. FMT resulted in rapid normalization of bacterial fecal sample composition from a markedly dysbiotic state to one representative of normal fecal microbiota. While the microbiome appeared most similar to the donor implant material 1 day post-FMT, the composition diverged variably at later time points. The donor microbiota composition also varied over time. However, both post-FMT and donor samples remained within the larger cloud of fecal microbiota characterized as healthy by the HMP.
Conclusions: Dynamic behavior is an intrinsic property of normal fecal microbiota and should be accounted for in comparing microbial communities among normal individuals and those with disease states. This also suggests that more frequent sample analyses are needed in order to properly assess success of FMT procedures.
Keywords: Short- and long-term changes in microbiota following FMT.
Figures
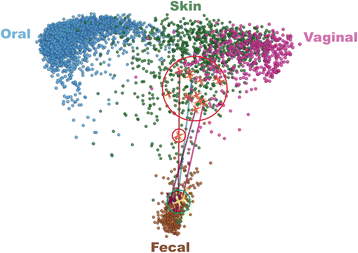
Figure 1
Fecal bacterial communities of recurrent CDI patients shift towards HMP fecal bacterial communities after FMT. Pre-FMT patient samples (red circle); post-FMT patient samples (green circles); trajectory of patient fecal communities after FMT (blue line).
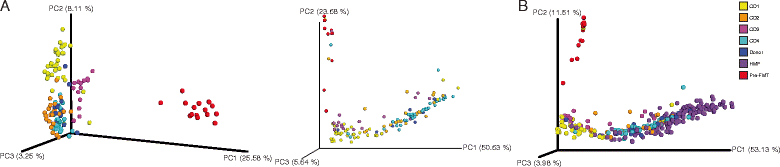
Figure 2
Microbial communities shift following FMT. (A) Unweighted (left) and weighted (right) UniFrac analyses followed by principal component analysis of bacterial communities of recurrent CDI patient fecal samples before (red) and after FMT and donor samples (blue). (B) Weighted UniFrac analysis followed by principal component analysis of bacterial communities of patients before (red) and after FMT versus HMP fecal communities (purple). PC, principal component. Percentages represent percent variability explained by each principal component. Se key at right for colors associated with samples before FMT (pre-FMT), from HMP and donor, and from patients after FMT (CD1 to CD4).
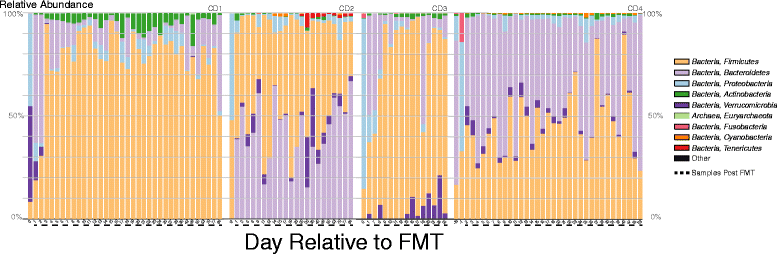
Figure 3
Changes in fecal microbial communities after FMT. Relative abundance of sequences classified to the level of bacterial phyla before and after FMT in patient fecal samples. Samples after FMT indicated with dashed line. See key at right.
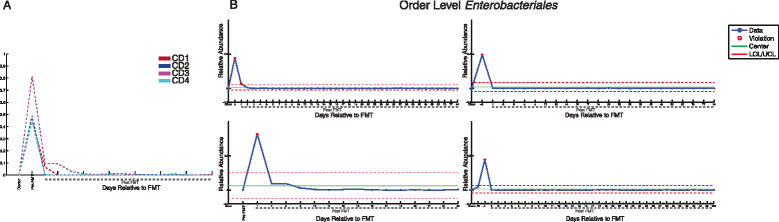
Figure 4
Changes in the order Enterobacteriales after FMT. (A) Relative abundance of Enterobacteriales in donor and patient samples before and after FMT in samples common across all patients. (B) Control charts of relative abundance of Enterobacteriales in donor (leftmost sample) and patient samples before and after FMT. Patient CD1 (top left), patient CD2 (top right), patient CD3 (bottom left), patient CD4 (bottom right). LCL, lower control limit; UCL, upper control limit; mean relative abundance in all samples (center). LCL and UCL represent three standard deviations in relative abundance below and above the mean, respectively. Dashed lines indicate samples after FMT.
Figure 5
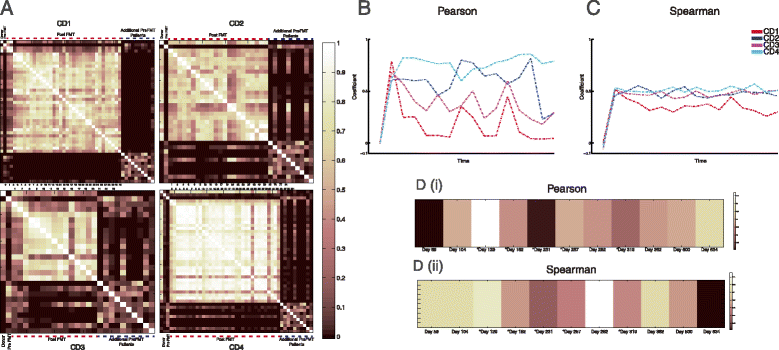
Pearson and Spearman correlations between fecal communities before and after FMT. (A) Heat map of Pearson correlation values between each sample within each patient set, corresponding donor, and 10 additional pre-FMT patient samples (far right). (B) Pearson correlation values between donor sample and each patient sample. (C) Spearman correlations between donor sample and each patient sample. (D) Heat maps of Pearson (i) and Spearman (ii) correlation values between earliest donor sample and eleven subsequent samples; days represent collection time of each sample versus earliest donor sample. CD1 to CD4, patients 1 to 4. Dashed lines indicate samples after FMT.
Similar articles
- Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection.
Weingarden AR, Chen C, Bobr A, Yao D, Lu Y, Nelson VM, Sadowsky MJ, Khoruts A. Weingarden AR, et al. Am J Physiol Gastrointest Liver Physiol. 2014 Feb 15;306(4):G310-9. doi: 10.1152/ajpgi.00282.2013. Epub 2013 Nov 27. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24284963 Free PMC article. - Predicting recurrence of Clostridium difficile infection following encapsulated fecal microbiota transplantation.
Staley C, Kaiser T, Vaughn BP, Graiziger CT, Hamilton MJ, Rehman TU, Song K, Khoruts A, Sadowsky MJ. Staley C, et al. Microbiome. 2018 Sep 18;6(1):166. doi: 10.1186/s40168-018-0549-6. Microbiome. 2018. PMID: 30227892 Free PMC article. - Successful therapy of Clostridium difficile infection with fecal microbiota transplantation.
Konturek PC, Koziel J, Dieterich W, Haziri D, Wirtz S, Glowczyk I, Konturek K, Neurath MF, Zopf Y. Konturek PC, et al. J Physiol Pharmacol. 2016 Dec;67(6):859-866. J Physiol Pharmacol. 2016. PMID: 28195066 - Microbial composition analysis of Clostridium difficile infections in an ulcerative colitis patient treated with multiple fecal microbiota transplantations.
Brace C, Gloor GB, Ropeleski M, Allen-Vercoe E, Petrof EO. Brace C, et al. J Crohns Colitis. 2014 Sep;8(9):1133-7. doi: 10.1016/j.crohns.2014.01.020. Epub 2014 Feb 13. J Crohns Colitis. 2014. PMID: 24529606 Review. - Faecal microbiota transplantation for Clostridium difficile infection.
Dodin M, Katz DE. Dodin M, et al. Int J Clin Pract. 2014 Mar;68(3):363-8. doi: 10.1111/ijcp.12320. Epub 2013 Dec 22. Int J Clin Pract. 2014. PMID: 24372725 Review.
Cited by
- An ecological framework to understand the efficacy of fecal microbiota transplantation.
Xiao Y, Angulo MT, Lao S, Weiss ST, Liu YY. Xiao Y, et al. Nat Commun. 2020 Jul 3;11(1):3329. doi: 10.1038/s41467-020-17180-x. Nat Commun. 2020. PMID: 32620839 Free PMC article. - Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection.
Jalanka J, Mattila E, Jouhten H, Hartman J, de Vos WM, Arkkila P, Satokari R. Jalanka J, et al. BMC Med. 2016 Oct 11;14(1):155. doi: 10.1186/s12916-016-0698-z. BMC Med. 2016. PMID: 27724956 Free PMC article. - Qiita: rapid, web-enabled microbiome meta-analysis.
Gonzalez A, Navas-Molina JA, Kosciolek T, McDonald D, Vázquez-Baeza Y, Ackermann G, DeReus J, Janssen S, Swafford AD, Orchanian SB, Sanders JG, Shorenstein J, Holste H, Petrus S, Robbins-Pianka A, Brislawn CJ, Wang M, Rideout JR, Bolyen E, Dillon M, Caporaso JG, Dorrestein PC, Knight R. Gonzalez A, et al. Nat Methods. 2018 Oct;15(10):796-798. doi: 10.1038/s41592-018-0141-9. Epub 2018 Oct 1. Nat Methods. 2018. PMID: 30275573 Free PMC article. - GePMI: A statistical model for personal intestinal microbiome identification.
Wang Z, Lou H, Wang Y, Shamir R, Jiang R, Chen T. Wang Z, et al. NPJ Biofilms Microbiomes. 2018 Sep 4;4:20. doi: 10.1038/s41522-018-0065-2. eCollection 2018. NPJ Biofilms Microbiomes. 2018. PMID: 30210803 Free PMC article. - Patchouli alcohol attenuates the cognitive deficits in a transgenic mouse model of Alzheimer's disease via modulating neuropathology and gut microbiota through suppressing C/EBPβ/AEP pathway.
Xu QQ, Su ZR, Yang W, Zhong M, Xian YF, Lin ZX. Xu QQ, et al. J Neuroinflammation. 2023 Jan 30;20(1):19. doi: 10.1186/s12974-023-02704-1. J Neuroinflammation. 2023. PMID: 36717922 Free PMC article.
References
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–60. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources