A proteomic analysis reveals the interaction of GluK1 ionotropic kainate receptor subunits with Go proteins - PubMed (original) (raw)
A proteomic analysis reveals the interaction of GluK1 ionotropic kainate receptor subunits with Go proteins
Izabela Rutkowska-Wlodarczyk et al. J Neurosci. 2015.
Abstract
Kainate receptors (KARs) are found ubiquitously in the CNS and are present presynaptically and postsynaptically regulating synaptic transmission and excitability. Functional studies have proven that KARs act as ion channels as well as potentially activating G-proteins, thus indicating the existance of a dual signaling system for KARs. Nevertheless, it is not clear how these ion channels activate G-proteins and which of the KAR subunits is involved. Here we performed a proteomic analysis to define proteins that interact with the C-terminal domain of GluK1 and we identified a variety of proteins with many different functions, including a Go α subunit. These interactions were verified through distinct in vitro and in vivo assays, and the activation of the Go protein by GluK1 was validated in bioluminescence resonance energy transfer experiments, while the specificity of this association was confirmed in GluK1-deficient mice. These data reveal components of the KAR interactome, and they show that GluK1 and Go proteins are natural partners, accounting for the metabotropic effects of KARs.
Keywords: GluK1; Go protein; kainate receptors; metabotropic; noncanonical; proteomics.
Copyright © 2015 the authors 0270-6474/15/355171-09$15.00/0.
Figures
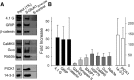
Figure 1.
Assessing the GluK1 receptor interactome in pull-down assays. A, Recombinant protein (S-GluK1b C-terminal or control construct) bound to S-protein agarose was incubated with a mouse brain homogenate to pull-down interacting proteins, which were studied in immunoblots with the antibodies indicated. B, Average densitometry quantification from three independent experiments and, depending on the enrichment, the proteins may be divided into three groups. Note that Gαo appears as a protein that clearly interacts with the GluK1 subunit.
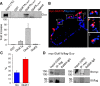
Figure 2.
Colocalization of myc-GluK1b and Gαo in SH-SY5Y cells. A, GST-based pull-down revealed that Gαo specifically interacts with the C-terminal domain of GluK1b. Measurements are the mean ± SEM from six (GST, GluK1a, and GluK5) and eight (GluK5b) different pull-down experiments and are referenced against GST band density. B, Colocalization of membrane GluK1b and Gαo in SH-SY5Y cells transfected with both proteins. Confocal images show the overlapping immunofluorescence of flag-Gαo (blue) and myc-GluK1b (red) in cotransfected cells. C, Quantification of these images show that 25.9 ± 4.9% of the blue dots (flag-Gαo) colocalize or lie in close proximity to the red dots (myc-GluK1b, extracellularly labeled), and 58.2 ± 3.25% of red dots colocalize with blue dots. The data are the mean ± SEM of measurements from 15 cells. D, The Gαo subunit coimmunoprecipitates GluK1b. Extracts from SH-SY5Y cells cotransfected with myc-GluK1b and flag-Gαo were immunoprecipitated (IP) with a rabbit anti-flag, anti-myc, or appropriate IgG antibody and probed in Western blots (IB) with anti-myc or anti-flag antibodies as indicated.
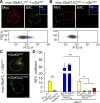
Figure 3.
Gαo interacts with GluK1 subunits in a cell system. A, HEK293 cells transfected with the myc-GluK1b and Gαo fusion proteins to which the C-terminal (VCT) and N-terminal (VNT) domains of the Venus YFP variant had been respectively added. Single confocal images showing the immunolabeling of extracellular myc (Myc, red) and intracellular Gαo (Go, blue), as well as the reconstitution of Venus fluorescence (yellow). B, Removal of the C-terminal domain of GluK1 (GluK1ΔCVCT) selectively prevented the fluorescence complementation in cells showing normal levels of the expressed proteins. Dot plots of the FACS analysis are shown below A and B. These represent the fluorescence intensity (exponential arbitrary units, FITC-A) against cell complexity (FSC-H). Gray and blue dots represent the nonfluorescent and fluorescent cell populations, respectively (100,000 cells sorted in each case). C, BiFC in cells transfected with GαoVCT, myc-GluK1b, and myc-GluK5VNT. Note how fluorescence accumulates intracellularly when GluK1 and GluK5VNT form heterodimers (top, arrow), a phenomenon that disappears upon removal of the C-terminal domain of GluK5 (bottom). D, Quantification of BiFC by FACS under different experimental conditions. The data are the mean ± SEM of four independent experiments, each performed in triplicate. *p < 0.05, **p < 0.01 (1-way repeated-measures ANOVA, Tukey's test).
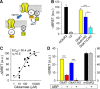
Figure 4.
GluK1 activates Gαo proteins. A, Diagram representing the BRET assay used to study activation of a heterotrimeric G-protein by GluK1 receptors in living cells. Gαo and γ2 subunits are fused with the energy donor (Rluc) and acceptor (YFP), respectively. Receptor activation generates the dissociation of the G-protein complex and the loss of energy transfer. B, BRET signal variation in cells transfected with the indicated receptor upon perfusion of glutamate (1 m
m
). C, Dose–response curve for glutamate in the GluK1-induced loss of the BRET signal. D, The KAR antagonist UBP310 (100 μ
m
) inhibits the activation of heterotrimeric Gαo/βγ complex by GluK1 but not by mGluR2 receptors. The data in B and D are the mean ± SEM of three independent experiments, each performed in triplicate. *p < 0.05, ***p < 0.001 (Student's t test).
Figure 5.
Deletion of GluK1 but not GluK5 prevents noncanonical signaling of kainate receptors. A, application of kainate (100 n
m
) reduces the peak _I_AHP amplitude in wild-type DRG neurons but not in those dissociated from GluK1−/− mice. Sample traces (top) before (black) and after (gray) application of kainate. Bottom, Time course of kainate-evoked inhibition in neurons from control (black) and GluK1−/− mice (open symbols). B, Proportion of cells in which inhibition was observed in several experimental conditions (top) and the inhibition in neurons from GluK1 and GluK5 wild-type and deficient mice. Numbers within bars are the number of studied neurons. ***p < 0.001 (Student's t test).
Similar articles
- Determination of kainate receptor subunit ratios in mouse brain using novel chimeric protein standards.
Watanabe-Iida I, Konno K, Akashi K, Abe M, Natsume R, Watanabe M, Sakimura K. Watanabe-Iida I, et al. J Neurochem. 2016 Jan;136(2):295-305. doi: 10.1111/jnc.13384. Epub 2015 Oct 30. J Neurochem. 2016. PMID: 26448475 - Neto2 Assembles with Kainate Receptors in DRG Neurons during Development and Modulates Neurite Outgrowth in Adult Sensory Neurons.
Vernon CG, Swanson GT. Vernon CG, et al. J Neurosci. 2017 Mar 22;37(12):3352-3363. doi: 10.1523/JNEUROSCI.2978-16.2017. Epub 2017 Feb 24. J Neurosci. 2017. PMID: 28235897 Free PMC article. - Phosphorylation of the kainate receptor (KAR) auxiliary subunit Neto2 at serine 409 regulates synaptic targeting of the KAR subunit GluK1.
Lomash RM, Sheng N, Li Y, Nicoll RA, Roche KW. Lomash RM, et al. J Biol Chem. 2017 Sep 15;292(37):15369-15377. doi: 10.1074/jbc.M117.787903. Epub 2017 Jul 17. J Biol Chem. 2017. PMID: 28717010 Free PMC article. - In the developing hippocampus kainate receptors control the release of GABA from mossy fiber terminals via a metabotropic type of action.
Cherubini E, Caiati MD, Sivakumaran S. Cherubini E, et al. Adv Exp Med Biol. 2011;717:11-26. doi: 10.1007/978-1-4419-9557-5_2. Adv Exp Med Biol. 2011. PMID: 21713663 Review. - Physiopathology of kainate receptors in epilepsy.
Crépel V, Mulle C. Crépel V, et al. Curr Opin Pharmacol. 2015 Feb;20:83-8. doi: 10.1016/j.coph.2014.11.012. Epub 2014 Dec 13. Curr Opin Pharmacol. 2015. PMID: 25506747 Review.
Cited by
- Unbalanced dendritic inhibition of CA1 neurons drives spatial-memory deficits in the Ts2Cje Down syndrome model.
Valbuena S, García Á, Mazier W, Paternain AV, Lerma J. Valbuena S, et al. Nat Commun. 2019 Nov 1;10(1):4991. doi: 10.1038/s41467-019-13004-9. Nat Commun. 2019. PMID: 31676751 Free PMC article. - Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert.
Reiner A, Levitz J. Reiner A, et al. Neuron. 2018 Jun 27;98(6):1080-1098. doi: 10.1016/j.neuron.2018.05.018. Neuron. 2018. PMID: 29953871 Free PMC article. Review. - Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels.
Hansen KB, Wollmuth LP, Bowie D, Furukawa H, Menniti FS, Sobolevsky AI, Swanson GT, Swanger SA, Greger IH, Nakagawa T, McBain CJ, Jayaraman V, Low CM, Dell'Acqua ML, Diamond JS, Camp CR, Perszyk RE, Yuan H, Traynelis SF. Hansen KB, et al. Pharmacol Rev. 2021 Oct;73(4):298-487. doi: 10.1124/pharmrev.120.000131. Pharmacol Rev. 2021. PMID: 34753794 Free PMC article. Review. - Kainate Receptors Inhibit Glutamate Release Via Mobilization of Endocannabinoids in Striatal Direct Pathway Spiny Projection Neurons.
Marshall JJ, Xu J, Contractor A. Marshall JJ, et al. J Neurosci. 2018 Apr 18;38(16):3901-3910. doi: 10.1523/JNEUROSCI.1788-17.2018. Epub 2018 Mar 14. J Neurosci. 2018. PMID: 29540547 Free PMC article. - Splicing and editing of ionotropic glutamate receptors: a comprehensive analysis based on human RNA-Seq data.
Herbrechter R, Hube N, Buchholz R, Reiner A. Herbrechter R, et al. Cell Mol Life Sci. 2021 Jul;78(14):5605-5630. doi: 10.1007/s00018-021-03865-z. Epub 2021 Jun 8. Cell Mol Life Sci. 2021. PMID: 34100982 Free PMC article.
References
- Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous