White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke - PubMed (original) (raw)
White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke
Jun Suenaga et al. Exp Neurol. 2015 Oct.
Abstract
Most of the successes in experimental models of stroke have not translated well to the clinic. One potential reason for this failure is that stroke mainly afflicts the elderly and the majority of experimental stroke studies rely on data gathered from young adult animals. Therefore, in the present study we established a reliable, reproducible model of stroke with low mortality in aged (18month) male mice and contrasted their pathophysiological changes with those in young (2month) animals. To this end, mice were subjected to permanent tandem occlusion of the left distal middle cerebral artery (dMCAO) with ipsilateral common carotid artery occlusion (CCAO). Cerebral blood flow (CBF) was evaluated repeatedly during and after stroke. Reduction of CBF was more dramatic and sustained in aged mice. Aged mice exhibited more severe long-term sensorimotor deficits, as manifested by deterioration of performance in the Rotarod and hanging wire tests up to 35d after stroke. Aged mice also exhibited significantly worse long-term cognitive deficits after stroke, as measured by the Morris water maze test. Consistent with these behavioral observations, brain infarct size and neuronal tissue loss after dMCAO were significantly larger in aged mice at 2d and 14d, respectively. The young versus aged difference in neuronal tissue loss, however, did not persist until 35d after dMCAO. In contrast to the transient difference in neuronal tissue loss, we found significant and long lasting deterioration of white matter in aged animals, as revealed by the loss of myelin basic protein (MBP) staining in the striatum at 35d after dMCAO. We further examined the expression of M1 (CD16/CD32) and M2 (CD206) markers in Iba-1(+) microglia by double immunofluorescent staining. In both young and aged mice, the expression of M2 markers peaked around 7d after stroke whereas the expression of M1 markers peaked around 14d after stroke, suggesting a progressive M2-to-M1 phenotype shift in both groups. However, aged mice exhibited significantly reduced M2 polarization compared to young adults. Remarkably, we discovered a strong positive correlation between favorable neurological outcomes after dMCAO and MBP levels or the number of M2 microglia/macrophages. In conclusion, our studies suggest that the distal MCAO stroke model consistently results in ischemic brain injury with long-term behavioral deficits, and is therefore suitable for the evaluation of long-term stroke outcomes. Furthermore, aged mice exhibit deterioration of functional outcomes after stroke and this deterioration is linked to white matter damage and reductions in M2 microglia/macrophage polarization.
Keywords: Aging; Cerebral blood perfusion; Distal middle cerebral artery occlusion (dMCAO); Microglia.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
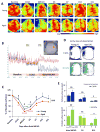
Fig. 1. Aged mice exhibit reduced cerebral reperfusion over time after distal MCAO
Cerebral blood flow (CBF) was monitored using the 2 dimensional laser speckle technique at 0d (baseline, after CCAO, and after distal MCAO), 3d, 7d, 14d, and 21d after distal MCAO. A. Representative 2-D laser speckle images in young and aged groups. Images represent the axial surface view from the top. The left side of the picture designates the left infarct region. B. Representative recordings of CBF in young animals before and 0d after dMCAO. The inset shows two recording areas in the infarct region (blue) and the counterpart on the contralateral side (red). C. CBF in the infarct region was quantified and expressed as percent change from baseline (pre-dMCAO). Data are means ± SEM. n=4 per group. *_p_≤0.05, ****_p_≤0.0001 vs corresponding baseline. #_p_≤0.05, ### _p_≤0.001, young vs aged. D. Representative images of surface areas where the CBF decreased to less than 30% (blue), or between 30-50% (green) of baseline at 0d after dMCAO. E. Quantification of surface areas where the CBF decreased to less than 30% (blue), or between 30-50% (green) of baseline at 0d, 3d and 14d after dMCAO. Data are means ± SEM. n=4 per group. # p ≤0.05; ## p ≤0.01; NS, not significant.
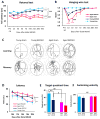
Fig. 2. Aged mice show greater deterioration in sensorimotor and cognitive functions after dMCAO
A-B. Sensorimotor functions were evaluated up to 35d after dMCAO or sham operation in young and aged mice. n=8/group. Shown are the mean ± SEM. A. Rotarod test. *** _p_≤0.001, **** _p_≤0.0001, #### _p_≤0.0001. B. Hanging wire test. **_p_≤0.01, ***_p_≤0.001 vs corresponding sham, # _p_≤0.05, ###_p_≤0.001 vs. young dMCAO. C-F. Long-term cognitive functions were assessed by the Morris water maze. n=8/group. Shown are the mean ± SEM. C. Representative images of the swim paths of mice in each group while the platform was present (learning phase) and after it was removed (memory phase). D. More severe learning deficits were observed in aged mice, as reflected by longer escape time. **_p_≤0.01, **** _p_≤0.0001 vs corresponding sham. E. More severe memory deficits were observed in aged mice, as reflected by shorter time spent in the target quadrant. ** _p_≤0.01, # _p_≤0.05, ## _p_≤0.01. F. Both groups had similar swim speeds, thereby showing equivalent motor skills. ns: no significance.
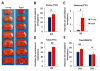
Fig. 3. Distal MCAO results in larger brain infarct volumes soon after stroke in aged mice compared to young adult mice
A. Representative TTC staining at 2d after distal MCAO in young and aged mice. Black dotted line in brain sections from aged mice designates the additional infarct area in the striatum. B-D. Quantification of infarct volume at 2d after dMCAO in young and aged mice. Infarct sizes were expressed as percentages of the contralateral hemisphere in the cortex (B), striatum (C) and total hemisphere (D). Values are means ± SEM. n=4 per group. #_p_≤0.05, ##_p_≤0.01. B-D, Quantification of brain tissue loss at 14d and 35d after dMCAO in young and aged mice, as determined by immunostaining for the neuronal marker MAP2. Values are means ± SEM. n=4 per group. ##_p_≤0.01; ns: no significance.
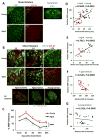
Fig. 4. White matter injury in aged mice is linked with functional deficits at late stages of dMCAO
A. MBP and SMI-32 staining in sham-operated young and aged mice. White squares in lower magnification views of MBP staining designate the area shown under high magnification on the left. EC, external capsule. B. Representative images of MBP and SMI-32 double-staining at 1, 3, and 14d after dMCAO in young and aged mice. Scale bar: 100 μm. High power images of the striatum at 3d after dMCAO were shown in lower panels. Scale bar: 50 μm. C. Quantification of MBP staining intensities in the striatum in young and aged mice after sham-operation or at 1, 3, 14, and 35 days after dMCAO. Data were expressed as percentage of young sham. n=7 per group. *_p_≤0.05, **_p_≤0.01, vs corresponding sham. ####_p_≤0.0001 young vs aged. D-E. Pearson correlation between MBP staining intensity and behavioral performance on the Rotarod or Morris water maze memory tests. D. There was a positive correlation between MBP staining intensity and Rotarod performance at 35d after dMCAO. E. There was a positive correlation between MBP intensity and water maze performance at 21d after dMCAO. F-G. Pearson correlation between MBP and SMI-32 staining intensity. F. There was a negative correlation between SMI-32 intensity and MBP intensity in aged mice after dMCAO. G. There was no significant correlation between SMI-32 intensity and MBP intensity in young mice after dMCAO.
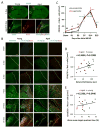
Fig. 5. M1 microglia/macrophage polarization after dMCAO in young and aged mice
A. Image analysis of microglia/macrophage markers in 3 ipsilateral regions (square boxes) at the inner boundary of the infarct. The boundary of the infarct was identified by the loss of MAP2 staining (upper panel) and the boundary of GFAP+ glial scar (lower panel). Scale bar: 40 μm. The letter C represents the ischemic core. B. Representative images of CD16/32 (M1 marker; green) and Iba1 (microglia marker; red) double staining at 1, 3, 7, 14, and 35d after dMCAO or in sham-operated animals. Dotted line designates the infarct borderline. Scale bar: 120 μm. C. Quantification of the number of CD16/32+Iba1+ cells in the ischemic border zone in young adult and aged mice. Values are means ± SEM. n=4 per group. * _p_≤0.05, **_p_≤0.01, ***_p_≤0.001, ****_p_≤0.0001 vs corresponding sham. #_p_≤0.05, ##_p_≤0.01 young vs aged. D-E. Lack of correlation between the number of M1 cells and behavioral performance on the Rotarod at 35d after dMCAO or the Morris water maze memory test at 21d after dMCAO.
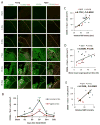
Fig. 6. M2 microglia/macrophage polarization after dMCAO in young and aged mice
A. Representative images of CD206 (M2 marker; green) and Iba1 (red) double staining at 1, 3, 7, 14, and 35d after dMCAO or in sham-operated animals. Dotted line designates the infarct borderline. Scale bar: 120 μm. B. Quantification of the number of CD206+Iba1+ cells in the ischemic border zone in young adult and aged mice. Values are means ± SEM. n=4 per group. *_p_≤0.05, **_p_≤0.01, ***_p_≤0.001, vs corresponding sham. # _p_≤0.05, ## _p_≤0.01 young vs aged. C-D. Pearson correlation between the number of M2 cells and behavioral performance on the Rotarod or Morris water maze memory tests. C. There was a positive correlation between the number of M2 microglia/macrophage and Rotarod performance at 35d after dMCAO. D. There was a positive correlation between the number of M2 microglia/macrophage and water maze performance at 21d after dMCAO. E. There was a positive correlation between the number of M2 microglia/macrophage and MBP staining intensity at 35d after dMCAO.
Similar articles
- Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery After Cerebral Ischemia.
Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, Ji X, Leak RK, Gao Y, Chen J, Hu X. Liu X, et al. Stroke. 2016 Feb;47(2):498-504. doi: 10.1161/STROKEAHA.115.012079. Epub 2016 Jan 5. Stroke. 2016. PMID: 26732561 Free PMC article. - A Post-stroke Therapeutic Regimen with Omega-3 Polyunsaturated Fatty Acids that Promotes White Matter Integrity and Beneficial Microglial Responses after Cerebral Ischemia.
Jiang X, Pu H, Hu X, Wei Z, Hong D, Zhang W, Gao Y, Chen J, Shi Y. Jiang X, et al. Transl Stroke Res. 2016 Dec;7(6):548-561. doi: 10.1007/s12975-016-0502-6. Epub 2016 Oct 7. Transl Stroke Res. 2016. PMID: 27714669 Free PMC article. - Brain-selective mild hypothermia promotes long-term white matter integrity after ischemic stroke in mice.
Liu LQ, Liu XR, Zhao JY, Yan F, Wang RL, Wen SH, Wang L, Luo YM, Ji XM. Liu LQ, et al. CNS Neurosci Ther. 2018 Dec;24(12):1275-1285. doi: 10.1111/cns.13061. Epub 2018 Sep 16. CNS Neurosci Ther. 2018. PMID: 30295998 Free PMC article. - Post-stroke administration of omega-3 polyunsaturated fatty acids promotes neurovascular restoration after ischemic stroke in mice: Efficacy declines with aging.
Jiang X, Suenaga J, Pu H, Wei Z, Smith AD, Hu X, Shi Y, Chen J. Jiang X, et al. Neurobiol Dis. 2019 Jun;126:62-75. doi: 10.1016/j.nbd.2018.09.012. Epub 2018 Sep 12. Neurobiol Dis. 2019. PMID: 30218758 Review. - Treatment targets for M2 microglia polarization in ischemic stroke.
Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Wang J, et al. Biomed Pharmacother. 2018 Sep;105:518-525. doi: 10.1016/j.biopha.2018.05.143. Epub 2018 Jun 6. Biomed Pharmacother. 2018. PMID: 29883947 Review.
Cited by
- Soluble epoxide hydrolase inhibition enhances anti-inflammatory and antioxidative processes, modulates microglia polarization, and promotes recovery after ischemic stroke.
Yeh CF, Chuang TY, Hung YW, Lan MY, Tsai CH, Huang HX, Lin YY. Yeh CF, et al. Neuropsychiatr Dis Treat. 2019 Oct 15;15:2927-2941. doi: 10.2147/NDT.S210403. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 31686827 Free PMC article. - Paclitaxel induces cognitive impairment via necroptosis, decreased synaptic plasticity and M1 polarisation of microglia.
Tang M, Zhao S, Liu JX, Liu X, Guo YX, Wang GY, Wang XL. Tang M, et al. Pharm Biol. 2022 Dec;60(1):1556-1565. doi: 10.1080/13880209.2022.2108064. Pharm Biol. 2022. PMID: 35944285 Free PMC article. - DRα1-MOG-35-55 Reduces Permanent Ischemic Brain Injury.
Wang J, Ye Q, Xu J, Benedek G, Zhang H, Yang Y, Liu H, Meza-Romero R, Vandenbark AA, Offner H, Gao Y. Wang J, et al. Transl Stroke Res. 2017 Jun;8(3):284-293. doi: 10.1007/s12975-016-0514-2. Epub 2016 Dec 17. Transl Stroke Res. 2017. PMID: 27988839 Free PMC article. - Regulatory microRNAs and vascular cognitive impairment and dementia.
Zhang J, Sun P, Zhou C, Zhang X, Ma F, Xu Y, Hamblin MH, Yin KJ. Zhang J, et al. CNS Neurosci Ther. 2020 Dec;26(12):1207-1218. doi: 10.1111/cns.13472. CNS Neurosci Ther. 2020. PMID: 33459504 Free PMC article. Review. - The Neural Cell Adhesion Molecule-Derived (NCAM)-Peptide FG Loop (FGL) Mobilizes Endogenous Neural Stem Cells and Promotes Endogenous Regenerative Capacity after Stroke.
Klein R, Mahlberg N, Ohren M, Ladwig A, Neumaier B, Graf R, Hoehn M, Albrechtsen M, Rees S, Fink GR, Rueger MA, Schroeter M. Klein R, et al. J Neuroimmune Pharmacol. 2016 Dec;11(4):708-720. doi: 10.1007/s11481-016-9694-5. Epub 2016 Jun 28. J Neuroimmune Pharmacol. 2016. PMID: 27352075
References
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. - PMC - PubMed
- Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS43802/NS/NINDS NIH HHS/United States
- NS45048/NS/NINDS NIH HHS/United States
- R01 NS089534/NS/NINDS NIH HHS/United States
- R01 NS095671/NS/NINDS NIH HHS/United States
- R01 NS043802/NS/NINDS NIH HHS/United States
- I01 BX002495/BX/BLRD VA/United States
- I01 RX000420/RX/RRD VA/United States
- NS089534/NS/NINDS NIH HHS/United States
- R01 NS036736/NS/NINDS NIH HHS/United States
- R01 NS045048/NS/NINDS NIH HHS/United States
- NS36736/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous