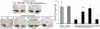Exosomes as drug delivery vehicles for Parkinson's disease therapy - PubMed (original) (raw)
Exosomes as drug delivery vehicles for Parkinson's disease therapy
Matthew J Haney et al. J Control Release. 2015.
Erratum in
- Corrigendum to "Exosomes as drug delivery vehicles for Parkinson's disease therapy" [Journal of Controlled Release 207, (2015) 18-30].
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Haney MJ, et al. J Control Release. 2021 Nov 10;339:232-234. doi: 10.1016/j.jconrel.2021.09.027. Epub 2021 Oct 2. J Control Release. 2021. PMID: 34610512 No abstract available.
Abstract
Exosomes are naturally occurring nanosized vesicles that have attracted considerable attention as drug delivery vehicles in the past few years. Exosomes are comprised of natural lipid bilayers with the abundance of adhesive proteins that readily interact with cellular membranes. We posit that exosomes secreted by monocytes and macrophages can provide an unprecedented opportunity to avoid entrapment in mononuclear phagocytes (as a part of the host immune system), and at the same time enhance delivery of incorporated drugs to target cells ultimately increasing drug therapeutic efficacy. In light of this, we developed a new exosomal-based delivery system for a potent antioxidant, catalase, to treat Parkinson's disease (PD). Catalase was loaded into exosomes ex vivo using different methods: the incubation at room temperature, permeabilization with saponin, freeze-thaw cycles, sonication, or extrusion. The size of the obtained catalase-loaded exosomes (exoCAT) was in the range of 100-200nm. A reformation of exosomes upon sonication and extrusion, or permeabilization with saponin resulted in high loading efficiency, sustained release, and catalase preservation against proteases degradation. Exosomes were readily taken up by neuronal cells in vitro. A considerable amount of exosomes was detected in PD mouse brain following intranasal administration. ExoCAT provided significant neuroprotective effects in in vitro and in vivo models of PD. Overall, exosome-based catalase formulations have a potential to be a versatile strategy to treat inflammatory and neurodegenerative disorders.
Keywords: Blood–brain barrier; Catalase; Exosomes; Neuroinflammation; Oxidative stress; Parkinson's disease.
Published by Elsevier B.V.
Figures
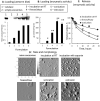
Figure 1. Characterization of exoCAT
Exosomes released from Raw 264.7 macrophages were loaded with catalase by different techniques and examined by: (A) western blot, (B) catalase 10 enzymatic activity; and (C) catalase release. ExoCAT morphology was examined by AFM (D).
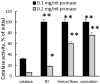
Figure 2. Preservation of catalase enzymatic activity in exoCAT
ExoCAT obtained by sonication demonstrated the best protection of catalase.
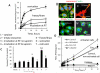
Figure 3. Accumulation of exoCAT in PC12 cells, and therapeutic effects of exoCAT in in vitro models of oxidative stress
The exoCAT uptake in PC12 cells was examined by spectrofluorimetry (A), and confocal microscopy (B). The bar: 20 µm. The neuroprotection by exoCAT formulations was evaluated in the cell pre-incubated with 6-OHDA (C); (1) catalase, (2) empty exosomes, catalase loaded into exosomes by: (3) incubation at RT, (4) saponin permeabilization, (5) freeze/thaw cycles, (6) sonication, (7) extrusion. The ability to decrease levels of ROS produced in activated macrophages (pre-incubated with LPS and TNF-α) by exoCAT was evaluated by Ampex Red assay in vitro (D).
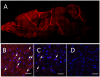
Figure 4. Biodistribution of DIL-labeled exosomes in mouse brain
Exosomes were administered to mice with 6-OHDA-induced brain inflammation through: intranasal (A, B), or intravenous (C) routs; and compared to PBS-injected controls (D). The bar: 40 µm.
Figure 5. Anti-inflammatory effects of exoCAT in PD mouse model
The intranasal administration of exoCAT significantly decreased microglial activation (D, E) in 6-OHDA-intoxicated mice compared to those intoxicated with 6-OHDA and then treated with PBS (C). Catalase alone did not decrease inflammation in PD mice (F). Empty exosomes did not alter the microglial status in healthy animals (B) compared to healthy controls (A). The anti-inflammatory effects of the described exosomal formulations were quantified by the amount of activated microglial cells (G).
Figure 6. Neuroprotective effects of exoCAT in PD mouse model
The i.n. administration of exoCAT protected DA neurons (D, E) in 6-OHDA-intoxicated mice compared to those intoxicated with 6-OHDA and then treated with PBS (C). Catalase alone was not efficient in this model (F). Empty exosomes did not affect the number of DA neurons in healthy animals (B) compared to healthy controls (A) indicating the absence of cytotoxic effects of the exosomal carriers. The neuroprotective effects of the described exosomal formulations were quantified by the amount of DA neurons in the SNpc (G).
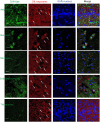
Figure 7. Co-localization of exosomes with different cells in the mouse brain with inflammation
Exosomes released by BMM were labeled with DIL (red). C57BL/6 mice were intoxicated with 6-OHDA, and then i.n. injected with fluorescently-labeled exosomes. Four hours later, mice were sacrificed, perfused, and brain slides were subjected for confocal examinations. Brain slides were stained with antibodies to different cell types and then secondary ab 594 (green). Nucleus was stained with DAPI (blue). Bar: 10 µm.
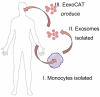
Figure 8. The flow of exoCAT formulations in clinic
Similar articles
- Oil based nanocarrier system for transdermal delivery of ropinirole: a mechanistic, pharmacokinetic and biochemical investigation.
Azeem A, Talegaonkar S, Negi LM, Ahmad FJ, Khar RK, Iqbal Z. Azeem A, et al. Int J Pharm. 2012 Jan 17;422(1-2):436-44. doi: 10.1016/j.ijpharm.2011.10.039. Epub 2011 Oct 25. Int J Pharm. 2012. PMID: 22057087 - Delivery of Dual Drug Loaded Lipid Based Nanoparticles across the Blood-Brain Barrier Impart Enhanced Neuroprotection in a Rotenone Induced Mouse Model of Parkinson's Disease.
Kundu P, Das M, Tripathy K, Sahoo SK. Kundu P, et al. ACS Chem Neurosci. 2016 Dec 21;7(12):1658-1670. doi: 10.1021/acschemneuro.6b00207. Epub 2016 Oct 3. ACS Chem Neurosci. 2016. PMID: 27642670 - inPentasomes: An innovative nose-to-brain pentamidine delivery blunts MPTP parkinsonism in mice.
Rinaldi F, Seguella L, Gigli S, Hanieh PN, Del Favero E, Cantù L, Pesce M, Sarnelli G, Marianecci C, Esposito G, Carafa M. Rinaldi F, et al. J Control Release. 2019 Jan 28;294:17-26. doi: 10.1016/j.jconrel.2018.12.007. Epub 2018 Dec 7. J Control Release. 2019. PMID: 30529726 - Nanoparticle technology for treatment of Parkinson's disease: the role of surface phenomena in reaching the brain.
Leyva-Gómez G, Cortés H, Magaña JJ, Leyva-García N, Quintanar-Guerrero D, Florán B. Leyva-Gómez G, et al. Drug Discov Today. 2015 Jul;20(7):824-37. doi: 10.1016/j.drudis.2015.02.009. Epub 2015 Feb 17. Drug Discov Today. 2015. PMID: 25701281 Review. - Micro- and nanotechnology approaches to improve Parkinson's disease therapy.
Torres-Ortega PV, Saludas L, Hanafy AS, Garbayo E, Blanco-Prieto MJ. Torres-Ortega PV, et al. J Control Release. 2019 Feb 10;295:201-213. doi: 10.1016/j.jconrel.2018.12.036. Epub 2018 Dec 21. J Control Release. 2019. PMID: 30579984 Review.
Cited by
- Long non-coding RNAs: From disease code to drug role.
Chen Y, Li Z, Chen X, Zhang S. Chen Y, et al. Acta Pharm Sin B. 2021 Feb;11(2):340-354. doi: 10.1016/j.apsb.2020.10.001. Epub 2020 Oct 10. Acta Pharm Sin B. 2021. PMID: 33643816 Free PMC article. Review. - Separation, characterization, and standardization of extracellular vesicles for drug delivery applications.
Buschmann D, Mussack V, Byrd JB. Buschmann D, et al. Adv Drug Deliv Rev. 2021 Jul;174:348-368. doi: 10.1016/j.addr.2021.04.027. Epub 2021 May 5. Adv Drug Deliv Rev. 2021. PMID: 33964356 Free PMC article. Review. - Roles of exosomes in regenerative periodontology: a narrative review.
Koca-Ünsal RB, Chaurasia A. Koca-Ünsal RB, et al. Mol Biol Rep. 2022 Dec;49(12):12219-12225. doi: 10.1007/s11033-022-08010-y. Epub 2022 Oct 20. Mol Biol Rep. 2022. PMID: 36266554 Review. - Cell-cell communication: new insights and clinical implications.
Su J, Song Y, Zhu Z, Huang X, Fan J, Qiao J, Mao F. Su J, et al. Signal Transduct Target Ther. 2024 Aug 7;9(1):196. doi: 10.1038/s41392-024-01888-z. Signal Transduct Target Ther. 2024. PMID: 39107318 Free PMC article. Review. - The emerging role of exosomes as novel therapeutics: Biology, technologies, clinical applications, and the next.
Song Y, Kim Y, Ha S, Sheller-Miller S, Yoo J, Choi C, Park CH. Song Y, et al. Am J Reprod Immunol. 2021 Feb;85(2):e13329. doi: 10.1111/aji.13329. Epub 2020 Sep 12. Am J Reprod Immunol. 2021. PMID: 32846024 Free PMC article. Review.
References
- Mcgeer PL, Itagaki S, Boyes BE, Mcgeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38(8):1285–1291. - PubMed
- Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995;378(6559):776–779. - PubMed
- Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in Parkinson's disease. Prog Neurobiol. 1996;48(1):1–19. - PubMed
- Ambani LM, Van Woert MH, Murphy S. Brain peroxidase and catalase in Parkinson disease. Arch Neurol. 1975;32(2):114–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous