3',4',5',5,7-pentamethoxyflavone sensitizes Cisplatin-resistant A549 cells to Cisplatin by inhibition of Nrf2 pathway - PubMed (original) (raw)
3',4',5',5,7-pentamethoxyflavone sensitizes Cisplatin-resistant A549 cells to Cisplatin by inhibition of Nrf2 pathway
Xiangyu Hou et al. Mol Cells. 2015 May.
Abstract
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important redox-sensitive transcription factor that regulates the expression of several cytoprotective genes. More recently, genetic analyses of human tumors have indicated that Nrf2 may cause resistance to chemotherapy. In this study, we found that the expression levels of Nrf2 and its target genes GCLC, HO-1, NQO1 were significantly higher in cisplatin-resistant A549 (A549/CDDP) cells than those in A549 cells, and this resistance was partially reversed by Nrf2 siRNA. 3',4',5',5,7-Pentamethoxyflavone (PMF), a natural flavonoid extracted from Rutaceae plants, sensitized A549/CDDP to CDDP and substantially induced apoptosis compared with that of CDDP alone treated group, and this reversal effect decreased when Nrf2 was downregulated by siRNA. Mechanistically, PMF reduced Nrf2 expression leading to a reduction of Nrf2 downstream genes, and in contrast, this effect was decreased by blocking Nrf2 with siRNA. Taken together, these results demonstrated that PMF could be used as an effective adjuvant sensitizer to increase the efficacy of chemotherapeutic drugs by downregulating Nrf2 signaling pathway.
Keywords: 3′,4′,5′,5,7-Pentamethoxyflavone; Nrf2; chemoresistance; cisplatin; lung cancer.
Figures
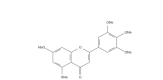
Fig. 1.
Chemical structure of 3′,4′,5′,5,7-Pentamethoxyflavone (PMF)
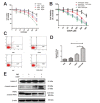
Fig. 2.
Nrf2 mediated defense was upregulated in A549/CDDP cells than in A549 cells. (A) The inhibitory effect of cisplatin on the viability of A549 and A549/CDDP cells. Cells were treated with series concentration of CDDP for 48 h and then viabilities were determined by SRB assay. (B–C) The expression of nuclear and total protein of Nrf2 and its target proteins GCLC, HO-1, NQO1 was assessed by western blot in A549 and A549/CDDP cells. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
Fig. 3.
PMF sensitized A549/CDDP cells to CDDP. (A) A549/CDDP cells were exposed to PMF (10, 25, and 50 μM) and were incubated with increasing concentration of CDDP (5 – 400 μM) in culture for 48 h and SRB assay were taken. (B) Comparison of the suppression of PMF (25 μM) combined with different concentration of CDDP on A549 cells and A549/CDDP cells. (C–D) Apoptosis ratios were determined through flow cytometry. (E) The expression of cleaved Caspase 3 and PARP1were determined by Western blot. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
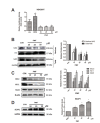
Fig. 4.
PMF inhibited Nrf2 signalling pathway. (A) HEK 293T cells were transfected with PGL3-ARE and pRL-TK plasmids., Twenty four hours after the transfection, the cells were treated with PMF (10, 25 and 50 μM) or tBHQ (10 μM) for 24 h. (B–D) A549/CDDP cells were treated with PMF (0–50 μM) for 24 hours. After treatment, nuclear fractions and total proteins were analyzed by western blot assay. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
Fig. 5.
PMF reduced the expression of p-Nrf2 and p-ERK in A549/CDDP cells. After A549/CDDP cells were treated with PMF (10, 25 and 50 μM) for 48 h, the expression of p-Nrf2 (A) and protein kinases ERK (B) were determined using Western blot analysis. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
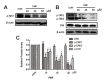
Fig. 6.
PMF increased the sensitivity of A549/CDDP cells to CDDP by inhibition of Nrf2. (A–B) Effect of Nrf2 knockdown on the sensitivity to CDDP. Cells were treated with CDDP (10 – 400 μM) alone or combination with 50 μM PMF for 48 h. Cell viability was determined by SRB assay. (C–D) Effect of Nrf2 knockdown on the expression of Nrf2-mediated antioxidant genes. *P < 0.05, versus resistance. Data are presented as means ± SD of three independent experiments and significant differences are indicated as *P < 0.05.
Similar articles
- Osthole promotes the suppressive effects of cisplatin on NRF2 expression to prevent drug-resistant cervical cancer progression.
Su J, Zhang F, Li X, Liu Z. Su J, et al. Biochem Biophys Res Commun. 2019 Jun 25;514(2):510-517. doi: 10.1016/j.bbrc.2019.04.021. Epub 2019 May 2. Biochem Biophys Res Commun. 2019. PMID: 31056260 - Cryptotanshinone Reverses Cisplatin Resistance of Human Lung Carcinoma A549 Cells through Down-Regulating Nrf2 Pathway.
Xia C, Bai X, Hou X, Gou X, Wang Y, Zeng H, Huang M, Jin J. Xia C, et al. Cell Physiol Biochem. 2015;37(2):816-24. doi: 10.1159/000430398. Epub 2015 Sep 11. Cell Physiol Biochem. 2015. PMID: 26356271 - Ginsenoside Rd reverses cisplatin resistance in non-small-cell lung cancer A549 cells by downregulating the nuclear factor erythroid 2-related factor 2 pathway.
Chian S, Zhao Y, Xu M, Yu X, Ke X, Gao R, Yin L. Chian S, et al. Anticancer Drugs. 2019 Sep;30(8):838-845. doi: 10.1097/CAD.0000000000000781. Anticancer Drugs. 2019. PMID: 31415285 - An overview of chemical inhibitors of the Nrf2-ARE signaling pathway and their potential applications in cancer therapy.
Zhu J, Wang H, Chen F, Fu J, Xu Y, Hou Y, Kou HH, Zhai C, Nelson MB, Zhang Q, Andersen ME, Pi J. Zhu J, et al. Free Radic Biol Med. 2016 Oct;99:544-556. doi: 10.1016/j.freeradbiomed.2016.09.010. Epub 2016 Sep 12. Free Radic Biol Med. 2016. PMID: 27634172 Review. - Nrf2 signaling pathway in cisplatin chemotherapy: Potential involvement in organ protection and chemoresistance.
Mirzaei S, Mohammadi AT, Gholami MH, Hashemi F, Zarrabi A, Zabolian A, Hushmandi K, Makvandi P, Samec M, Liskova A, Kubatka P, Nabavi N, Aref AR, Ashrafizadeh M, Khan H, Najafi M. Mirzaei S, et al. Pharmacol Res. 2021 May;167:105575. doi: 10.1016/j.phrs.2021.105575. Epub 2021 Mar 24. Pharmacol Res. 2021. PMID: 33771701 Review.
Cited by
- Nrf2 Inhibitor, Brusatol in Combination with Trastuzumab Exerts Synergistic Antitumor Activity in HER2-Positive Cancers by Inhibiting Nrf2/HO-1 and HER2-AKT/ERK1/2 Pathways.
Yang Y, Tian Z, Guo R, Ren F. Yang Y, et al. Oxid Med Cell Longev. 2020 Jul 19;2020:9867595. doi: 10.1155/2020/9867595. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32765809 Free PMC article. - Proteasome Inhibitor-Induced IκB/NF-κB Activation is Mediated by Nrf2-Dependent Light Chain 3B Induction in Lung Cancer Cells.
Lee KH, Lee J, Woo J, Lee CH, Yoo CG. Lee KH, et al. Mol Cells. 2018 Dec 31;41(12):1008-1015. doi: 10.14348/molcells.2018.0277. Epub 2018 Nov 6. Mol Cells. 2018. PMID: 30396235 Free PMC article. - The Essential Role of H19 Contributing to Cisplatin Resistance by Regulating Glutathione Metabolism in High-Grade Serous Ovarian Cancer.
Zheng ZG, Xu H, Suo SS, Xu XL, Ni MW, Gu LH, Chen W, Wang LY, Zhao Y, Tian B, Hua YJ. Zheng ZG, et al. Sci Rep. 2016 May 19;6:26093. doi: 10.1038/srep26093. Sci Rep. 2016. PMID: 27193186 Free PMC article. - Strange Bedfellows: Nuclear Factor, Erythroid 2-Like 2 (Nrf2) and Hypoxia-Inducible Factor 1 (HIF-1) in Tumor Hypoxia.
Toth RK, Warfel NA. Toth RK, et al. Antioxidants (Basel). 2017 Apr 6;6(2):27. doi: 10.3390/antiox6020027. Antioxidants (Basel). 2017. PMID: 28383481 Free PMC article. Review. - Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells.
Minaei A, Sabzichi M, Ramezani F, Hamishehkar H, Samadi N. Minaei A, et al. Mol Biol Rep. 2016 Feb;43(2):99-105. doi: 10.1007/s11033-016-3942-x. Epub 2016 Jan 9. Mol Biol Rep. 2016. PMID: 26748999
References
- Boesch-Saadatmandi C, Wagner AE, Graeser AC, Hundhausen C, Wolffram S, Rimbach G. Ochratoxin A impairs Nrf2-dependent gene expression in porcine kidney tubulus cells. J. Anim. Physiol. Anim. Nutr. 2009;93:547–554. - PubMed
- Cai H, Sale S, Schmid R, Britton RG, Brown K, Steward WP, Gescher AJ. Flavones as colorectal cancer chemopreventive agents--phenol-o-methylation enhances efficacy. Cancer Prev. Res. 2009;2:743–750. - PubMed
- Chen F, Liu Y, Wang S, Guo X, Shi P, Wang W, Xu B. Triptolide, a Chinese herbal extract, enhances drug sensitivity of resistant myeloid leukemia cell lines through downregulation of HIF-1a and Nrf2. Pharmacogenomics. 2013;14:1305–1317. - PubMed
- Chian S, Li YY, Wang XJ, Tang XW. Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugsvia inhibition of the Nrf2 pathway. Asian Pac. J. Cancer Prev. 2014;15:2911–2916. - PubMed
- D’Addario G, Felip E, ESMO Guidelines Working Group Non-small-cell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009;20:68–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous