Gas-phase intermolecular phosphate transfer within a phosphohistidine phosphopeptide dimer - PubMed (original) (raw)
Gas-phase intermolecular phosphate transfer within a phosphohistidine phosphopeptide dimer
Maria-Belen Gonzalez-Sanchez et al. Int J Mass Spectrom. 2014.
Abstract
The hydrogen bonds and electrostatic interactions that form between the protonated side chain of a basic residue and the negatively charged phosphate of a phosphopeptide can play crucial roles in governing their dissociation pathways under low-energy collision-induced dissociation (CID). Understanding how phosphoramidate (i.e. phosphohistidine, phospholysine and phosphoarginine), rather than phosphomonoester-containing peptides behave during CID is paramount in investigation of these problematic species by tandem mass spectrometry. To this end, a synthetic peptide containing either phosphohistidine (pHis) or phospholysine (pLys) was analyzed by ESI-MS using a Paul-type ion trap (AmaZon, Bruker) and by traveling wave ion mobility-mass spectrometry (Synapt G2-S_i_, Waters). Analysis of the products of low-energy CID demonstrated formation of a doubly 'phosphorylated' product ion arising from intermolecular gas-phase phosphate transfer within a phosphopeptide dimer. The results are explained by the formation of a homodimeric phosphohistidine (pHis) peptide non-covalent complex (NCX), likely stabilized by the electrostatic interaction between the pHis phosphate group and the protonated _C_-terminal lysine residue of the peptide. To the best of our knowledge this is the first report of intermolecular gas-phase phosphate transfer from one phosphopeptide to another, leading to a doubly phosphorylated peptide product ion.
Keywords: CID; Gas-phase dimer; Histidine phosphorylation; NCX, non-covalent complex; Non-covalent interactions; Phosphoramidate; Phosphotransfer; TWIMS, travelling wave ion mobility-mass spectrometry; pArg, phosphoarginine; pHis, phosphohistidine; pLys, phospholysine.
Figures
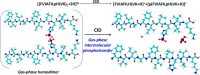
Graphical abstract
Fig. 1
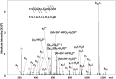
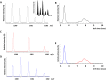
ESI-ion trap full scan mass spectrum of the products of the reaction between peptide FVIAFILHLVK and potassium phosphoramidate (KNH2PO3H2). Inset shows an enhanced region of the mass spectrum encompassing m/z range 1280–1400.
Fig. 2
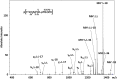
CID product ion mass spectrum of the doubly charged ion of the phosphorylated peptide p[FVIAFILHLVK+2H]2+ at m/z 690.5, indicating a heterogeneous population of [FVIAFILpHLVK+2H]2+ and [FVIAFILHLVpK+2H]2+, whose specific y-ions are labeled in italics (gray). (Δ) Loss of 80 Da (HPO3); (*) Observation of both phosphorylated and non-phosphorylated product ions.
Fig. 3
CID product ion mass spectrum of the singly charged ion of the phosphorylated peptide [p(FVIAFILHLVK)+H]+ at m/z 1379.5. (Δ) Loss of 80 Da (HPO3).
Fig. 4
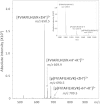
Traveling wave ion mobility-mass spectrometry analysis of the ions at m/z 1379.5 demonstrating a mixed population of singly protonated phosphopeptide monomer [M+H]+ and doubly protonated dimer [M2+2H]2+. (A) Isotopic distribution and (B) arrival time distribution (ATD) of the mixed population. Inset (A) depicts the theoretical isotope distribution (assuming 1:1 stoichiometry) of the [M+H]+ and [M2+2H]2+. Extracted ion current for the (C) longer (red) and (D) shorter (blue) ATDs (E) indicating mobility separation of the monomeric and dimeric populations.
Fig. 5
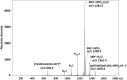
MS3 CID product ion mass spectrum of ions at m/z 1459.4 generated by CID of ions at m/z 1379.5. The doubly ‘phosphorylated’ peptide is represented by p(FVIAFILpHLVK). (Δ) Loss of 80 Da (HPO3).
Scheme 1
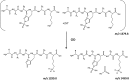
Proposed mechanism for the formation of the ions at m/z 1459.8. The scheme depicts a homodimer of the phosphopeptide FVIAFILpHLVK (m/z 1379.8), whose components can undergo elimination of metaphosphoric acid HPO3 and generation of a transient ternary complex, which then evolves to give the dephosphorylated peptide at m/z 1299.8 and the ‘doubly’ phosphorylated peptide at m/z 1459.8.
Similar articles
- Phosphohistidine phosphatase 1 (PHPT1) also dephosphorylates phospholysine of chemically phosphorylated histone H1 and polylysine.
Ek P, Ek B, Zetterqvist Ö. Ek P, et al. Ups J Med Sci. 2015 Mar;120(1):20-7. doi: 10.3109/03009734.2014.996720. Epub 2015 Jan 9. Ups J Med Sci. 2015. PMID: 25574816 Free PMC article. - A phosphohistidine proteomics strategy based on elucidation of a unique gas-phase phosphopeptide fragmentation mechanism.
Oslund RC, Kee JM, Couvillon AD, Bhatia VN, Perlman DH, Muir TW. Oslund RC, et al. J Am Chem Soc. 2014 Sep 17;136(37):12899-911. doi: 10.1021/ja507614f. Epub 2014 Sep 8. J Am Chem Soc. 2014. PMID: 25156620 Free PMC article. - Gas-Phase Rearrangement in Lysine Phosphorylated Peptides During Electron-Transfer Dissociation Tandem Mass Spectrometry.
Bertran-Vicente J, Schümann M, Hackenberger CP, Krause E. Bertran-Vicente J, et al. Anal Chem. 2015 Jul 21;87(14):6990-4. doi: 10.1021/acs.analchem.5b01389. Epub 2015 Jun 29. Anal Chem. 2015. PMID: 26110354 - The many ways that nature has exploited the unusual structural and chemical properties of phosphohistidine for use in proteins.
Kalagiri R, Hunter T. Kalagiri R, et al. Biochem J. 2021 Oct 15;478(19):3575-3596. doi: 10.1042/BCJ20210533. Biochem J. 2021. PMID: 34624072 Free PMC article. Review. - A journey from phosphotyrosine to phosphohistidine and beyond.
Hunter T. Hunter T. Mol Cell. 2022 Jun 16;82(12):2190-2200. doi: 10.1016/j.molcel.2022.05.007. Epub 2022 Jun 1. Mol Cell. 2022. PMID: 35654043 Free PMC article. Review.
Cited by
- Phosphopeptide Fragmentation and Site Localization by Mass Spectrometry: An Update.
Potel CM, Lemeer S, Heck AJR. Potel CM, et al. Anal Chem. 2019 Jan 2;91(1):126-141. doi: 10.1021/acs.analchem.8b04746. Epub 2018 Dec 5. Anal Chem. 2019. PMID: 30457327 Free PMC article. Review. No abstract available. - Gaining Confidence in the Elusive Histidine Phosphoproteome.
Potel CM, Lin MH, Prust N, van den Toorn HWP, Heck AJR, Lemeer S. Potel CM, et al. Anal Chem. 2019 May 7;91(9):5542-5547. doi: 10.1021/acs.analchem.9b00734. Epub 2019 Apr 23. Anal Chem. 2019. PMID: 30969750 Free PMC article. - Label scrambling during CID of covalently labeled peptide ions.
Borotto NB, Degraan-Weber N, Zhou Y, Vachet RW. Borotto NB, et al. J Am Soc Mass Spectrom. 2014 Oct;25(10):1739-46. doi: 10.1007/s13361-014-0962-4. Epub 2014 Jul 24. J Am Soc Mass Spectrom. 2014. PMID: 25056863 Free PMC article. - Phosphoproteomics in the Age of Rapid and Deep Proteome Profiling.
Riley NM, Coon JJ. Riley NM, et al. Anal Chem. 2016 Jan 5;88(1):74-94. doi: 10.1021/acs.analchem.5b04123. Epub 2015 Nov 19. Anal Chem. 2016. PMID: 26539879 Free PMC article. Review. No abstract available. - Ion/ion Charge Inversion/Attachment in Conjunction with Dipolar DC Collisional Activation as a Selective Screen for Sulfo- and Phosphopeptides.
Shih M, McLuckey SA. Shih M, et al. Int J Mass Spectrom. 2019 Oct;444:116181. doi: 10.1016/j.ijms.2019.116181. Epub 2019 Jul 11. Int J Mass Spectrom. 2019. PMID: 37064606 Free PMC article.
References
- Engholm-Keller K., Larsen M.R. Technologies and challenges in large-scale phosphoproteomics. Proteomics. 2013;13:910–931. - PubMed
- Boehm M.E., Seidler J., Hahn B., Lehmann W.D. Site-specific degree of phosphorylation in proteins measured by liquid chromatography–electrospray mass spectrometry. Proteomics. 2012;12:2167–2178. - PubMed
- Boersema P.J., Mohammed S., Heck A.J.R. Phosphopeptide fragmentation and analysis by mass spectrometry. J. Mass Spectrom. 2009;44:861–878. - PubMed
- Zubarev R.A., Horn D.M., Fridriksson E.K., Kelleher N.L., Kruger N.A., Lewis M.A., Carpenter B.K., McLafferty F.W. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal. Chem. 2000;72:563–573. - PubMed
Grants and funding
- BB/F00561X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H007113/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources