One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the EDITION 1 12-month randomized trial, including 6-month extension - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.1111/dom.12472. Epub 2015 May 11.
Affiliations
- PMID: 25846721
- PMCID: PMC4676922
- DOI: 10.1111/dom.12472
Randomized Controlled Trial
One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the EDITION 1 12-month randomized trial, including 6-month extension
M C Riddle et al. Diabetes Obes Metab. 2015 Sep.
Abstract
Aims: To evaluate the maintenance of efficacy and safety of insulin glargine 300 U/ml (Gla-300) versus glargine 100 U/ml (Gla-100) in people with type 2 diabetes mellitus (T2DM) using basal plus meal-time insulin for 12 months in the EDITION 1 trial.
Methods: EDITION 1 was a multicentre, randomized, open-label, two-arm, phase IIIa study. Participants completing the initial 6-month treatment period continued to receive Gla-300 or Gla-100, as previously randomized, once daily for a further 6-month open-label extension phase. Changes in glycated haemoglobin (HbA1c) and fasting plasma glucose concentrations, insulin dose, hypoglycaemic events and body weight were assessed.
Results: Of 807 participants enrolled in the initial phase, 89% (359/404) assigned to Gla-300 and 88% (355/403) assigned to Gla-100 completed 12 months. Glycaemic control was sustained in both groups (mean HbA1c: Gla-300, 7.24%; Gla-100, 7.42%), with more sustained HbA1c reduction for Gla-300 at 12 months: least squares mean difference Gla-300 vs Gla-100: HbA1c -0.17 [95% confidence interval (CI) -0.30 to -0.05]%. The mean daily basal insulin dose at 12 months was 1.03 U/kg for Gla-300 and 0.90 U/kg for Gla-100. Lower percentages of participants had ≥1 confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemic event with Gla-300 than Gla-100 at any time of day [24 h; 86 vs 92%; relative risk 0.94 (95% CI 0.89-0.99)] and during the night [54 vs 65%; relative risk 0.84 (95% CI 0.75-0.94)], while the annualized rates of such hypoglycaemic events were similar. No between-treatment differences in adverse events were apparent.
Conclusion: During 12 months of treatment of T2DM requiring basal and meal-time insulin, glycaemic control was better sustained and fewer individuals reported hypoglycaemia with Gla-300 than with Gla-100. The mean basal insulin dose was higher with Gla-300 compared with Gla-100, but total numbers of hypoglycaemic events and overall tolerability did not differ between treatments.
Keywords: basal insulin; glycaemic control; insulin glargine; meal-time insulin.
© 2015 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
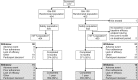
Figure 1
Participant flow diagram for EDITION 1. Upper portion with open boxes shows flow during the main 6-month study; shaded boxes below indicate flow during the extension phase up to 12 months. Gla-100, glargine 100 U/ml; Gla-300, glargine 300 U/ml; mITT, modified intention-to-treat. *During the main 6-month on-treatment period; †Not mutually exclusive with the reason for treatment discontinuation.
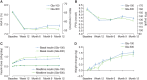
Figure 2
Clinical measures (mean ± standard error) during treatment by visit. (A) glycated haemoglobin (HbA1c) in the modified intention-to treat (mITT) population. (B) Laboratory-measured clinic-collected fasting plasma glucose (FPG) in the mITT population. (C) Daily basal insulin and meal-time insulin dose in the mITT population. (D) Weight change from baseline in the safety population. Gla-100, glargine 100 U/ml; Gla-300, glargine 300 U/ml.
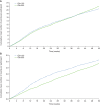
Figure 3
Cumulative mean numbers of confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemic events per participant during the 12-month study period (safety population). (A) Events occurring at any time of day (24 h). (B) Nocturnal events (00:00–05:59 hours). Gla-100, glargine 100 U/ml; Gla-300, glargine 300 U/ml [Correction added on May 29: In Figure 3A, the values of Gla-100 and Gla-300 were previously incorrect and these have now been amended in this version].
Similar articles
- New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 2).
Terauchi Y, Koyama M, Cheng X, Takahashi Y, Riddle MC, Bolli GB, Hirose T. Terauchi Y, et al. Diabetes Obes Metab. 2016 Apr;18(4):366-74. doi: 10.1111/dom.12618. Epub 2016 Jan 21. Diabetes Obes Metab. 2016. PMID: 26662838 Free PMC article. Clinical Trial. - Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes.
Ritzel R, Roussel R, Bolli GB, Vinet L, Brulle-Wohlhueter C, Glezer S, Yki-Järvinen H. Ritzel R, et al. Diabetes Obes Metab. 2015 Sep;17(9):859-67. doi: 10.1111/dom.12485. Epub 2015 Jun 16. Diabetes Obes Metab. 2015. PMID: 25929311 Free PMC article. - Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension.
Yki-Järvinen H, Bergenstal RM, Bolli GB, Ziemen M, Wardecki M, Muehlen-Bartmer I, Maroccia M, Riddle MC. Yki-Järvinen H, et al. Diabetes Obes Metab. 2015 Dec;17(12):1142-9. doi: 10.1111/dom.12532. Epub 2015 Sep 14. Diabetes Obes Metab. 2015. PMID: 26172084 Free PMC article. Clinical Trial. - Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes.
Ritzel R, Roussel R, Giaccari A, Vora J, Brulle-Wohlhueter C, Yki-Järvinen H. Ritzel R, et al. Diabetes Obes Metab. 2018 Mar;20(3):541-548. doi: 10.1111/dom.13105. Epub 2017 Oct 5. Diabetes Obes Metab. 2018. PMID: 28862801 Free PMC article. Review. - A review of the safety and efficacy data for insulin glargine 300 units/ml, a new formulation of insulin glargine.
Dailey G, Lavernia F. Dailey G, et al. Diabetes Obes Metab. 2015 Dec;17(12):1107-14. doi: 10.1111/dom.12531. Epub 2015 Sep 10. Diabetes Obes Metab. 2015. PMID: 26139151 Review.
Cited by
- Efficacy and safety of basal insulins in people with type 2 diabetes mellitus: a systematic review and network meta-analysis of randomized clinical trials.
Dehghani M, Sadeghi M, Barzkar F, Maghsoomi Z, Janani L, Motevalian SA, Loke YK, Ismail-Beigi F, Baradaran HR, Khamseh ME. Dehghani M, et al. Front Endocrinol (Lausanne). 2024 Mar 21;15:1286827. doi: 10.3389/fendo.2024.1286827. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38586456 Free PMC article. - 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024.
American Diabetes Association Professional Practice Committee. American Diabetes Association Professional Practice Committee. Diabetes Care. 2024 Jan 1;47(Suppl 1):S158-S178. doi: 10.2337/dc24-S009. Diabetes Care. 2024. PMID: 38078590 Review. - 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA, on behalf of the American Diabetes Association. ElSayed NA, et al. Diabetes Care. 2023 Jan 1;46(Suppl 1):S140-S157. doi: 10.2337/dc23-S009. Diabetes Care. 2023. PMID: 36507650 Free PMC article. Review. - Efficacy and safety of different basal and prandial insulin analogues for the treatment of type 2 diabetes: a network meta-analysis of randomized controlled trials.
Mannucci E, Caiulo C, Naletto L, Madama G, Monami M. Mannucci E, et al. Endocrine. 2021 Dec;74(3):508-517. doi: 10.1007/s12020-021-02889-6. Epub 2021 Oct 2. Endocrine. 2021. PMID: 34599695
References
- Bolli GB, Andreoli AM, Lucidi P. Optimizing the replacement of basal insulin in type 1 diabetes mellitus: no longer an elusive goal in the post-NPH era. Diabetes Technol Ther. 2011;13:S43–52. (Suppl. 1) - PubMed
- Tibaldi JM. Evolution of insulin development: focus on key parameters. Adv Ther. 2012;29:590–619. - PubMed
- Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26:S18–24. (Suppl. 3) - PubMed
- Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab. 2014;16:483–491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical