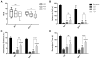Genetic deletion of the prostaglandin E2 E prostanoid receptor subtype 3 improves anatomical and functional outcomes after intracerebral hemorrhage - PubMed (original) (raw)
Genetic deletion of the prostaglandin E2 E prostanoid receptor subtype 3 improves anatomical and functional outcomes after intracerebral hemorrhage
Jenna L Leclerc et al. Eur J Neurosci. 2015 May.
Abstract
Intracerebral hemorrhage (ICH) is a stroke subtype associated with high mortality and morbidity. Following ICH, excitotoxicity and inflammation significantly contribute to secondary brain injury and poor outcomes. Prostaglandin E2 (PGE2 ) levels rise locally with insult to the nervous system, and PGE2 is known to modulate these processes mainly through its E prostanoid (EP) receptors, EP1-4. EP receptor subtype 3 (EP3) is the most abundant EP receptor in the brain and we have previously shown that signaling through the PGE2 -EP3 axis exacerbates excitotoxicity and ischemic stroke outcomes. This study aimed to investigate the contribution of this pathway in modulating anatomical outcomes and functional recovery following ICH. Genetic deletion of EP3 resulted in 48.2 ± 7.3% less ICH-induced brain injury (P < 0.005) and improved functional recovery (P < 0.05), as identified by neurological deficit scoring. To start investigating the mechanisms involved in neuroprotection with impaired PGE2 -EP3 signaling, histological staining was performed to evaluate blood and ferric iron accumulation, neuroinflammation, blood-brain barrier dysfunction, and peripheral neutrophil infiltration. After ICH, EP3 knockout mice demonstrated 49.5 ± 8.8% and 42.8 ± 13.1% less blood (P < 0.01) and ferric iron (P < 0.05), respectively. Furthermore, EP3 knockout mice had significantly reduced astrogliosis, microglial activation, blood-brain barrier breakdown, and neutrophil infiltration. Collectively, these results suggest an injurious role for the PGE2 -EP3 signaling axis in modulating brain injury, inflammation, and neurological functional recovery after ICH. Modulation of the PGE2 -EP3 signaling axis may represent a putative therapeutic avenue for the treatment of ICH.
Keywords: gliosis; iron; mouse; neuroinflammation; neuroprotection; stroke.
© 2015 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Fig. 1
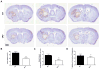
Genetic deletion of the PGE2 EP3 receptor attenuates ICH-induced brain injury. At 72 h after ICH, Cresyl violet staining of coronal brain sections from WT and EP3−/− mice was performed to evaluate brain injury. (A) Representative images showing characteristic hematoma profiles for WT (upper panels) and EP3−/− mice (bottom panels). Images are from the same animal, where left to right corresponds to anterior to posterior and center images are at the needle insertion site, representing maximal brain injury. (B) Lesion volume quantification demonstrated that EP3−/− mice had significantly less brain injury following ICH. (C) Red/brown positive pixel count analysis showed that EP3−/− mice had significantly less blood accumulation within the injured brain areas. (D) No significant difference in percent ipsilateral hemispheric enlargement was seen between the groups. All comparisons included n = 7 WT and n = 8 EP3−/− mice, ns = not significant and **p < 0.01.
Fig. 2
Effect of PGE2 EP3 receptor deletion on functional outcomes after ICH. At 24, 48, and 72 h following ICH, investigators blinded to genotype evaluated the mice for neurological deficits using several neurobehavioral tests. (A) EP3−/− mice had significant improvement in neurological function after ICH as identified by NDS, whereas no such recovery was seen for the WT group. (B) EP3−/− mice performed significantly worse at baseline on an accelerating rotarod. At 24, 48, and 72h after ICH, WT and EP3−/− mice had reduced latency to fall when compared to baseline function (p < 0.0001). No recovery in rotarod performance was seen for either group at any time point post-ICH. (C and D) No differences in baseline open field locomotor activity were seen for the WT and EP3−/− mice. Both groups had significantly impaired (C) ambulatory and (D) stereotypic movements at 24, 48, and 72 h post-ICH, when compared to baseline function (p < 0.0001). WT and EP3−/− mice had similar open field locomotor activity, where significant improvements in (C) ambulatory distance and (D) stereotypic time were seen at 72 h when compared to 24 h function. Similarly, both groups displayed improvements in (C) ambulatory distance at 72 h when compared to 48 h, but only EP3−/− mice had recovery of (D) stereotypic movements during this time frame. No differences in locomotor activity were seen between 24 and 48 h after ICH for either group. All comparisons included n = 7 WT and n = 9 EP3−/− mice. ns = not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, where the results above bars are with respect to 24 h function and brackets outline 48 to 72 h comparisons. ##p < 0.01 for comparison between WT and EP3−/− groups.
Fig. 3
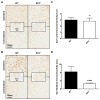
Genetic deletion of the PGE2 EP3 receptor reduces brain ferric iron Perls’ staining following ICH. At 72 h after ICH, Perls’ staining of coronal brain sections from WT and EP3−/− mice was performed to evaluate ferric iron content. (A) Representative high magnification images showing ferric iron accumulation (blue) in perihematomal regions from WT (left) and EP3−/− mice (right). Square selections in the inserts denote magnified regions. (B) Blue positive pixel count analysis of the ipsilateral hemisphere showed that EP3−/− mice had significantly less ferric iron deposition (WT: n = 7, EP3−/−: n = 8, *p < 0.05). No Perls’ staining was seen in the contralateral hemisphere for any of the mice in the study.
Fig. 4
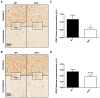
Effect of PGE2 EP3 receptor deletion on microgliosis after ICH. Immunohistochemical staining for Iba1 was used to evaluate cortical and striatal microglial activation and morphological changes in WT and EP3−/− mice 72 h following ICH. (A and B) Representative high magnification images of coronal brain sections showing the ipsilateral and contralateral (A) cortex and (B) striatum for WT (left) and EP3−/− mice (right). Square selections in the inserts denote magnified regions. (C and D) Quantification of brown positive pixel count demonstrated that WT and EP3−/− mice had similar (C) cortical Iba1 immunoreactivity, whereas EP3−/− mice displayed less (D) striatal Iba1 immunoreactivity. This reduced striatal microglial activation was accompanied by less morphological changes. Data is normalized to the corresponding contralateral equivalent signal, with appropriate control for the area of quantification in striatal analyses (see methods). All comparisons included n = 7 WT and n = 6 EP3−/− mice and ns = not significant.
Fig. 5
Effect of PGE2 EP3 receptor deletion on astrogliosis after ICH. Immunohistochemical staining for GFAP was used to evaluate cortical and striatal astrogliosis in WT and EP3−/− mice 72 h following ICH. (A and B) Representative high magnification images of coronal brain sections showing the ipsilateral and contralateral (A) cortex and (B) striatum for WT (left) and EP3−/− mice (right). Square selections in the inserts denote magnified regions. (C and D) Quantification of brown positive pixel count demonstrated that EP3−/− mice had significantly reduced (C) cortical GFAP immunoreactivity, and tended to have less (D) striatal astrogliosis. Both groups demonstrated negligible staining in the contralateral cortex and striatum; thus, data is presented as ipsilateral immunoreactivity corrected for the area of quantification, without normalization for the contralateral equivalent (see methods and supporting information). All comparisons included n = 7 WT and n = 8 EP3−/− mice and *p < 0.05.
Fig. 6
Genetic deletion of the PGE2 EP3 receptor reduces peripheral neutrophil infiltration after ICH. Immunohistochemical staining for MPO was used to evaluate peripheral neutrophil infiltration in WT and EP3−/− mice 72 h following ICH. (A) Representative high magnification images of coronal brain sections showing MPO+ cells (brown) diffusely localized within injured brain regions. Square selections in the inserts denote magnified regions. (B) Brown positive pixel count analysis of the ipsilateral hemisphere showed that EP3−/− mice had significantly less peripheral neutrophil infiltration (WT: n = 5, EP3−/−: n = 8, *p < 0.05). No MPO+ cells were seen outside of the injured brain areas for any of the mice in the study.
Fig. 7
Genetic deletion of the PGE2 EP3 receptor attenuates blood brain barrier breakdown after ICH. Immunohistochemical staining for mouse IgG was used to evaluate BBB breakdown in WT and EP3−/− mice at 72 h following ICH. (A) Representative images of coronal brain sections showing more disruption of the BBB in WT mice, which displayed a more diffuse staining pattern. Square boxes denote the location of high magnification inserts. (B) Whole brain brown positive pixel count analysis showed that EP3−/− mice had significantly reduced BBB breakdown (WT: n = 6, EP3−/−: n = 7, **p < 0.01).
Similar articles
- Intracerebral hemorrhage outcomes following selective blockade or stimulation of the PGE2 EP1 receptor.
Leclerc JL, Ahmad AS, Singh N, Soshnik-Schierling L, Greene E, Dang A, Doré S. Leclerc JL, et al. BMC Neurosci. 2015 Aug 1;16:48. doi: 10.1186/s12868-015-0182-2. BMC Neurosci. 2015. PMID: 26232001 Free PMC article. - PGE2-EP3 signaling exacerbates intracerebral hemorrhage outcomes in 24-mo-old mice.
Leclerc JL, Lampert AS, Diller MA, Doré S. Leclerc JL, et al. Am J Physiol Heart Circ Physiol. 2016 Jun 1;310(11):H1725-34. doi: 10.1152/ajpheart.00638.2015. Epub 2016 Apr 15. Am J Physiol Heart Circ Physiol. 2016. PMID: 27084388 Free PMC article. - Prostaglandin E2 EP2 receptor deletion attenuates intracerebral hemorrhage-induced brain injury and improves functional recovery.
Leclerc JL, Lampert AS, Diller MA, Immergluck JB, Doré S. Leclerc JL, et al. ASN Neuro. 2015 Apr 13;7(2):1759091415578713. doi: 10.1177/1759091415578713. Print 2015 Mar-Apr. ASN Neuro. 2015. PMID: 25873308 Free PMC article. - The Double Roles of the Prostaglandin E2 EP2 Receptor in Intracerebral Hemorrhage.
Luo X, Zhu Q, Zhang J, Huang Q, Xie Z, Cheng Y. Luo X, et al. Curr Drug Targets. 2017;18(12):1377-1385. doi: 10.2174/1389450117666151209122826. Curr Drug Targets. 2017. PMID: 26648067 Review. - The receptor EP3 to PGE2: A rational target to prevent atherothrombosis without inducing bleeding.
Mawhin MA, Tilly P, Fabre JE. Mawhin MA, et al. Prostaglandins Other Lipid Mediat. 2015 Sep;121(Pt A):4-16. doi: 10.1016/j.prostaglandins.2015.10.001. Epub 2015 Oct 14. Prostaglandins Other Lipid Mediat. 2015. PMID: 26463849 Review.
Cited by
- Intracerebral hemorrhage outcomes following selective blockade or stimulation of the PGE2 EP1 receptor.
Leclerc JL, Ahmad AS, Singh N, Soshnik-Schierling L, Greene E, Dang A, Doré S. Leclerc JL, et al. BMC Neurosci. 2015 Aug 1;16:48. doi: 10.1186/s12868-015-0182-2. BMC Neurosci. 2015. PMID: 26232001 Free PMC article. - Genetic Deletion of PGF2α-FP Receptor Exacerbates Brain Injury Following Experimental Intracerebral Hemorrhage.
Mohan S, Koller EJ, Fazal JA, De Oliveria G, Pawlowicz AI, Doré S. Mohan S, et al. Front Neurosci. 2018 Sep 5;12:556. doi: 10.3389/fnins.2018.00556. eCollection 2018. Front Neurosci. 2018. PMID: 30233287 Free PMC article. - Mechanism Study of the Protective Effects of Sodium Tanshinone IIA Sulfonate Against Atorvastatin-Induced Cerebral Hemorrhage in Zebrafish: Transcriptome Analysis.
Zhou ZY, Zhao WR, Xiao Y, Zhang J, Tang JY, Lee SM. Zhou ZY, et al. Front Pharmacol. 2020 Oct 2;11:551745. doi: 10.3389/fphar.2020.551745. eCollection 2020. Front Pharmacol. 2020. PMID: 33123006 Free PMC article. - A combination of serum iron, ferritin and transferrin predicts outcome in patients with intracerebral hemorrhage.
Yang G, Hu R, Zhang C, Qian C, Luo QQ, Yung WH, Ke Y, Feng H, Qian ZM. Yang G, et al. Sci Rep. 2016 Feb 22;6:21970. doi: 10.1038/srep21970. Sci Rep. 2016. PMID: 26898550 Free PMC article. - Leukotriene D4 and prostaglandin E2 signals synergize and potentiate vascular inflammation in a mast cell-dependent manner through cysteinyl leukotriene receptor 1 and E-prostanoid receptor 3.
Kondeti V, Al-Azzam N, Duah E, Thodeti CK, Boyce JA, Paruchuri S. Kondeti V, et al. J Allergy Clin Immunol. 2016 Jan;137(1):289-298. doi: 10.1016/j.jaci.2015.06.030. Epub 2015 Aug 5. J Allergy Clin Immunol. 2016. PMID: 26255103 Free PMC article.
References
- Ahmad AS, Saleem S, Ahmad M, Doré S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol Sci. 2006a;89:265–270. - PubMed
- Ahmad M, Saleem S, Zhuang H, Ahmad AS, Echeverria V, Sapirstein A, Doré S. 1-HydroxyPGE1 reduces infarction volume in mouse transient cerebral ischemia. Eur J Neurosci. 2006b;23:35–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 NS086441/NS/NINDS NIH HHS/United States
- R01 AG022971/AG/NIA NIH HHS/United States
- R01NS046400/NS/NINDS NIH HHS/United States
- R01 NS046400/NS/NINDS NIH HHS/United States
- F31NS086441/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous