Profiles of 21-Carbon Steroids in 21-hydroxylase Deficiency - PubMed (original) (raw)
Profiles of 21-Carbon Steroids in 21-hydroxylase Deficiency
Adina F Turcu et al. J Clin Endocrinol Metab. 2015 Jun.
Abstract
Context: Marked elevations of 17-hydroxyprogesterone (17OHP) are characteristic of classic 21-hydroxylase deficiency (21OHD). Testing of 17OHP provides the basis for 21OHD diagnosis, although it suffers from several pitfalls. False-positive or false-negative results and poor discrimination of nonclassic 21OHD from carriers limit the utility of serum 17OHP and necessitate dynamic testing after cosyntropin stimulation when values are indeterminate.
Objective: The objective was to provide a detailed characterization of 21-carbon (C21) steroids in classic 21OHD, which might identify other candidate steroids that could be employed for the diagnosis of 21OHD.
Setting and participants: Patients (11 women, 10 men) with classic 21OHD and 21 sex- and age-matched controls seen in a tertiary referral center were studied.
Methods: C21 steroids in the peripheral sera from all subjects, as well as in media from cultured testicular adrenal rest tumor (TART) cells and normal adrenal (NA) cells, were analyzed using liquid chromatography/tandem mass spectrometry (10 steroids). Additionally, the dynamics of C21 steroid metabolism in TART and NA cells were assessed with radiotracer studies.
Results: Five C21 steroids were significantly higher in 21OHD patients: 17OHP (67-fold; P < .01), 21-deoxycortisol (21dF; 35-fold; P < .01), 16α-hydroxyprogesterone (16OHP; 28-fold; P < .01), progesterone (2-fold; P < .01), and 11β-hydroxyprogesterone (11OHP; not detected in controls; P < .01). The same steroids were the highest in media from TART cells relative to the NA cells: 11OHP, 58- to 65-fold; 21dF, 30- to 41-fold; 17OHP, 9-fold; progesterone, 9- to 12-fold; and 16OHP, 7-fold.
Conclusion: Measurement of 16OHP and 11OHP along with 17OHP and 21dF by liquid chromatography/tandem mass spectrometry might comprise a biomarker panel to accurately diagnose all forms of 21OHD.
Figures
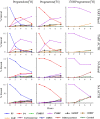
Figure 1.
Steroid metabolism in TART cells and normal adrenal (NA) cells. Cells were first treated with ACTH (10 nmol/L) or experimental medium for 48 hours. Immediately after media collection, cells were incubated with medium containing pregnenolone (P5), progesterone (P4), or 17OHP (1 μmol/L) and the corresponding [3H]labeled steroid (106 cpm/mL). Aliquots of medium were collected at 2, 4, and 8 hours. Samples were extracted, and radioactivity was quantified using HPLC. Data shown represent the percentage of total detected radiolabeled steroids (% steroid). 17OHP5, 17α-hydroxypregnenolone; 11DOC, 11-deoxycorticosterone.
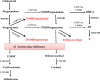
Figure 2.
Pathways of C21 steroids synthesis in 21OHD. HSD3B2, 3β-hydroxysteroid dehydrogenase type 2; CYP17A1, 17α-hydroxylase/17,20-lyase; CYB5A, cytochrome _b_5 type A; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; HSD17B5, 17β-hydroxysteroid dehydrogenase type 5.
Similar articles
- Superior discriminating value of ACTH-stimulated serum 21-deoxycortisol in identifying heterozygote carriers for 21-hydroxylase deficiency.
Costa-Barbosa FA, Tonetto-Fernandes VF, Carvalho VM, Nakamura OH, Moura V, Bachega TA, Vieira JG, Kater CE. Costa-Barbosa FA, et al. Clin Endocrinol (Oxf). 2010 Dec;73(6):700-6. doi: 10.1111/j.1365-2265.2010.03871.x. Clin Endocrinol (Oxf). 2010. PMID: 20846292 Clinical Trial. - Production of 11-Oxygenated Androgens by Testicular Adrenal Rest Tumors.
Schröder MAM, Turcu AF, O'Day P, van Herwaarden AE, Span PN, Auchus RJ, Sweep FCGJ, Claahsen-van der Grinten HL. Schröder MAM, et al. J Clin Endocrinol Metab. 2022 Jan 1;107(1):e272-e280. doi: 10.1210/clinem/dgab598. J Clin Endocrinol Metab. 2022. PMID: 34390337 Free PMC article. - Congenital Adrenal Hyperplasia: Time to Replace 17OHP with 21-Deoxycortisol.
Miller WL. Miller WL. Horm Res Paediatr. 2019;91(6):416-420. doi: 10.1159/000501396. Epub 2019 Aug 26. Horm Res Paediatr. 2019. PMID: 31450227 Review. - 21-hydroxylase deficiency transiently mimicking combined 21- and 11beta-hydroxylase deficiency.
Tonetto-Fernandes V, Lemos-Marini SH, De Mello MP, Ribeiro-Neto LM, Kater CE. Tonetto-Fernandes V, et al. J Pediatr Endocrinol Metab. 2008 May;21(5):487-94. doi: 10.1515/jpem.2008.21.5.487. J Pediatr Endocrinol Metab. 2008. PMID: 18655532 Review.
Cited by
- Cytochrome b5 Activates the 17,20-Lyase Activity of Human Cytochrome P450 17A1 by Increasing the Coupling of NADPH Consumption to Androgen Production.
Peng HM, Im SC, Pearl NM, Turcu AF, Rege J, Waskell L, Auchus RJ. Peng HM, et al. Biochemistry. 2016 Aug 9;55(31):4356-65. doi: 10.1021/acs.biochem.6b00532. Epub 2016 Jul 29. Biochemistry. 2016. PMID: 27426448 Free PMC article. - PRECISION MEDICINE IN ADRENAL DISORDERS: THE NEXT GENERATION.
Ghayee HK, Vinik AI, Pacak K; AACE Adrenal Scientific Committee. Ghayee HK, et al. Endocr Pract. 2017 Jun;23(6):672-679. doi: 10.4158/EP161716.RA. Epub 2017 Mar 23. Endocr Pract. 2017. PMID: 28332880 Free PMC article. Review. - The next 150 years of congenital adrenal hyperplasia.
Turcu AF, Auchus RJ. Turcu AF, et al. J Steroid Biochem Mol Biol. 2015 Sep;153:63-71. doi: 10.1016/j.jsbmb.2015.05.013. Epub 2015 Jun 3. J Steroid Biochem Mol Biol. 2015. PMID: 26047556 Free PMC article. Review. - Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency.
Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ. Turcu AF, et al. Eur J Endocrinol. 2016 May;174(5):601-9. doi: 10.1530/EJE-15-1181. Epub 2016 Feb 10. Eur J Endocrinol. 2016. PMID: 26865584 Free PMC article. - Inclusion of 11-Oxygenated Androgens in a Clinical Routine LC-MS/MS Setup for Steroid Hormone Profiling.
Zeidler R, Biemann R, Ceglarek U, Kratzsch J, Isermann B, Gaudl A. Zeidler R, et al. Int J Mol Sci. 2022 Dec 29;24(1):539. doi: 10.3390/ijms24010539. Int J Mol Sci. 2022. PMID: 36613983 Free PMC article.
References
- Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776–788. - PubMed
- Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, et al. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–590. - PubMed
- Therrell BL. Newborn screening for congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001;30:15–30. - PubMed
- White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245–291. - PubMed
- Pang S, Murphey W, Levine LS, et al. A pilot newborn screening for congenital adrenal hyperplasia in Alaska. J Clin Endocrinol Metab. 1982;55:413–420. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 DK069950/DK/NIDDK NIH HHS/United States
- R01DK069950/DK/NIDDK NIH HHS/United States
- DK089503/DK/NIDDK NIH HHS/United States
- R01 GM086596/GM/NIGMS NIH HHS/United States
- F32 DK103461/DK/NIDDK NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- R01GM086596/GM/NIGMS NIH HHS/United States
- U24 DK097153/DK/NIDDK NIH HHS/United States
- F32DK103461/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources