Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus - PubMed (original) (raw)
Review
Immunoregulatory cytokine networks: 60 years of learning from murine cytomegalovirus
Christine A Biron et al. Med Microbiol Immunol. 2015 Jun.
Abstract
Innate immunity defends against infection but also mediates immunoregulatory effects shaping innate and adaptive responses. Studies of murine cytomegalovirus (MCMV) infections have helped elucidate the mechanisms inducing, as well as the elicited soluble and cellular networks contributing to, innate immunity. Specialized receptors are engaged by infection-induced structures to stimulate production of key innate cytokines. These then stimulate cytokine and cellular responses such as activation of natural killer (NK) cells to mediate elevated killing by type 1 interferon (IFN) and/or to produce the pro-inflammatory and antiviral cytokine IFN-γ by interleukin 12 (IL-12). An inter-systemic loop, with IL-6 inducing glucocorticoid release, negatively regulates these early cytokine responses. As infections advance into periods of overlapping innate and adaptive responses, however, the cells are intrinsically conditioned to modify the biological effects of exposure to individual cytokines. Some pathways are turned off to inhibit an existing, whereas others are broadened for acquisition of a new, response function. Remarkably, extended NK cell proliferation during MCMV infection is associated with epigenetic modifications shifting the state of the inhibitory cytokine IL-10 gene from closed to open and results in their becoming equipped to produce this cytokine. When induced, NK cell IL-10 negatively regulates the magnitude of adaptive responses to protect against immune pathology. Thus, innate immunoregulatory cytokine networks are integral to pro-inflammatory and defense functions, but responding cells have the flexibility to undergo cell intrinsic conditioning with changing network characteristics to result in a new negative immunoregulatory function, and consequently, both promote beneficial and limit detrimental immune responses.
Figures
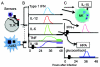
Figure 1
Induction of innate cytokine networks during MCMV infection. (A-B) Specialized sensors recognize viral products or induced virus-induced changes on/in infected cells to signal a threat. These are found on many cell types, but the TLRs and cytosolic recepotrs expressed on membranes in uninfected DCs and infected moncyte/macrophages survey the environment to stimulate the production of the type 1 IFNs, IL-12, TNF, and IL-6 during MCMV infections. A cytosolic AIM2 receptor is also stimulated in infected cells to induce the procession of biologically active IL-18. Once these cytokines are induced, they promote pro-inflammatory responses. (C) The type 1 IFNs have an important role in inducing elevated NK cell mediated killing, and IL-12 is a potent inducer of NK cell IFN-γ production. Once induced, IL-6 leads to HPA axis activation to stimulate glucocorticoid release and provide feedback inhibition of cytokine expression. (Based on the studies reported in [27,26,15,16]. Model modified from reference [4].)
Figure 2
Conditioning of NK cells during MCMV for changing NK cell function. (A) Serum measurements of IL-10 and IFN-γ by Cytometric Bead Assay during low (5,000 PFU) or high (70,000 PFU) dose infection with MCMV. (B) IL-10 production from media conditioned for 24h with total splenic leukocytes from day 0, uninfected, or 3.5 MCMV-infected mice, or with FACS-purified NK (CD49b+TCR-β-) and T (CD49b-TCRβ+) cells prepared from day 3.5 MCMV infection. (C) IL-10 expression, evaluated by induction of the IL-10-GFP reporter gene, among CD49b+TCRβ-NK cells taken from day 0, 1.5, 2.5, or 3.5 infected mice, in culture with IL-2, with or without IL-12, and with or without mitomycin C pre-treatment to block proliferation. (D) Distribution of histone methylation marks H3K4 (open), H3K27 (closed), and H3K36 (open) from purified NK cells on day 0, 1.5, and 3.5 of infection for the IFN-γ, IL-10, and Myod1 genes, as assessed by chromatin immunoprecipitation and massive parallel sequencing. (Figures reproduced with permission from reference [20]: panel A from Fig. 1A, 1F, panel B from Fig. 7B, and panel C from Fig. 6.)
Figure 3
A proliferation-dependent conditioning of NK cells to acquire the ability to express IL-10 and mediate negative immunoregulatory function. Because their IFN-γ gene has histone methylations in a configuration open for gene expression but those for the IL-10 gene in a closed configuration, NK cells in uninfected mice are initially prepared to respond to IL-12 with IFN-γ but not IL-10 production, and they do so at early times after MCMV infection. These events are occurring as the adaptive T cells responses are slowly being induced. Under conditions of extend and elevated MCMV replication, subsets of NK cells expressing the Ly49H activating receptor, which recognizes the m157 viral protein as its ligand, are stimulated to undergo preferential expansion. As a result of these events, the IL-10 gene is shifted from a closed to an open state for expression. Conditioned by their experiences, these NK cells now produce IL-10 during high dose infections to negatively regulated adaptive T cell responses, and can respond to IL-12 exposure with both IFN-γ and IL-10 production. Hence, proliferation promotes the flexible use of this innate cell type for pro-inflammatory/antiviral and negative immunoregulaotry functions as needed. (Based on the studies reported in reference [20].)
Similar articles
- Cytokine-Mediated Activation of NK Cells during Viral Infection.
Freeman BE, Raué HP, Hill AB, Slifka MK. Freeman BE, et al. J Virol. 2015 Aug;89(15):7922-31. doi: 10.1128/JVI.00199-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995253 Free PMC article. - CD8 T cells in innate immune responses: using STAT4-dependent but antigen-independent pathways to gamma interferon during viral infection.
Suarez-Ramirez JE, Tarrio ML, Kim K, Demers DA, Biron CA. Suarez-Ramirez JE, et al. mBio. 2014 Oct 21;5(5):e01978-14. doi: 10.1128/mBio.01978-14. mBio. 2014. PMID: 25336459 Free PMC article. - Calling in the troops: regulation of inflammatory cell trafficking through innate cytokine/chemokine networks.
Salazar-Mather TP, Hokeness KL. Salazar-Mather TP, et al. Viral Immunol. 2003;16(3):291-306. doi: 10.1089/088282403322396109. Viral Immunol. 2003. PMID: 14583145 Review. - Cytokine and chemokine networks: pathways to antiviral defense.
Salazar-Mather TP, Hokeness KL. Salazar-Mather TP, et al. Curr Top Microbiol Immunol. 2006;303:29-46. doi: 10.1007/978-3-540-33397-5_2. Curr Top Microbiol Immunol. 2006. PMID: 16570855 Review.
Cited by
- Direct antigen presentation is the canonical pathway of cytomegalovirus CD8 T-cell priming regulated by balanced immune evasion ensuring a strong antiviral response.
Büttner JK, Becker S, Fink A, Brinkmann MM, Holtappels R, Reddehase MJ, Lemmermann NA. Büttner JK, et al. Front Immunol. 2023 Dec 12;14:1272166. doi: 10.3389/fimmu.2023.1272166. eCollection 2023. Front Immunol. 2023. PMID: 38149242 Free PMC article. - Cytokine-Mediated Induction and Regulation of Tissue Damage During Cytomegalovirus Infection.
Clement M, Humphreys IR. Clement M, et al. Front Immunol. 2019 Jan 29;10:78. doi: 10.3389/fimmu.2019.00078. eCollection 2019. Front Immunol. 2019. PMID: 30761144 Free PMC article. Review. - CD8+ T Cells and NK Cells: Parallel and Complementary Soldiers of Immunotherapy.
Rosenberg J, Huang J. Rosenberg J, et al. Curr Opin Chem Eng. 2018 Mar;19:9-20. doi: 10.1016/j.coche.2017.11.006. Epub 2017 Dec 14. Curr Opin Chem Eng. 2018. PMID: 29623254 Free PMC article. - Interleukin 35 induced Th2 and Tregs bias under normal conditions in mice.
Zhang X, Zhang Z, He Z, Ju M, Li J, Yuan J, Jing Y, Li K, Liu Y, Li G. Zhang X, et al. PeerJ. 2018 Sep 21;6:e5638. doi: 10.7717/peerj.5638. eCollection 2018. PeerJ. 2018. PMID: 30258726 Free PMC article. - Human Cytomegalovirus Immediate-Early 1 Protein Rewires Upstream STAT3 to Downstream STAT1 Signaling Switching an IL6-Type to an IFNγ-Like Response.
Harwardt T, Lukas S, Zenger M, Reitberger T, Danzer D, Übner T, Munday DC, Nevels M, Paulus C. Harwardt T, et al. PLoS Pathog. 2016 Jul 7;12(7):e1005748. doi: 10.1371/journal.ppat.1005748. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27387064 Free PMC article.
References
- Smith MG. Propagation of salivary gland virus of the mouse in tissue cultures. Proc Soc Exp Biol Med. 1954;86(3):435–440. - PubMed
- Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor symposia on quantitative biology. 1989;54:1–13. Pt 1. - PubMed
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology. 2010;11(5):373–384. doi:10.1038/ni.1863. - PubMed
- Dalod M, Biron CA. Immunoregulatory cytokine networks discovered and characterized during murine cytomegalovirus infections. In: Reddenhase MJ, editor. CYTOMEGALOVIRUSES: From Molecular Pathogenesis to Therapy vol II. Caister Academic Press; 2013. pp. 232–258.
- Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21(1):107–119. doi:10.1016/j.immuni.2004.06.007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials