Influenza A Virus Protein PA-X Contributes to Viral Growth and Suppression of the Host Antiviral and Immune Responses - PubMed (original) (raw)
Influenza A Virus Protein PA-X Contributes to Viral Growth and Suppression of the Host Antiviral and Immune Responses
Tsuyoshi Hayashi et al. J Virol. 2015 Jun.
Abstract
Influenza virus infection causes global inhibition of host protein synthesis in infected cells. This host shutoff is thought to allow viruses to escape from the host antiviral response, which restricts virus replication and spread. Although the mechanism of host shutoff is unclear, a novel viral protein expressed by ribosomal frameshifting, PA-X, was found to play a major role in influenza virus-induced host shutoff. However, little is known about the impact of PA-X expression on currently circulating influenza A virus pathogenicity and the host antiviral response. In this study, we rescued a recombinant influenza A virus, A/California/04/09 (H1N1, Cal), containing mutations at the frameshift motif in the polymerase PA gene (Cal PA-XFS). Cal PA-XFS expressed significantly less PA-X than Cal wild type (WT). Cal WT, but not Cal PA-XFS, induced degradation of host β-actin mRNA and suppressed host protein synthesis, supporting the idea that PA-X induces host shutoff via mRNA decay. Moreover, Cal WT inhibited beta interferon (IFN-β) expression and replicated more rapidly than Cal PA-XFS in human respiratory cells. Mice infected with Cal PA-XFS had significantly lower levels of viral growth and greater expression of IFN-β mRNA in their lungs than mice infected with Cal WT. Importantly, more antihemagglutinin and neutralizing antibodies were produced in Cal PA-XFS-infected mice than in Cal WT-infected mice, despite the lower level of virus replication in the lungs. Our data indicate that PA-X of the pandemic H1N1 virus has a strong impact on viral growth and the host innate and acquired immune responses to influenza virus.
Importance: Virus-induced host protein shutoff is considered to be a major factor allowing viruses to evade innate and acquired immune recognition. We provide evidence that the 2009 H1N1 influenza A virus protein PA-X plays a role in virus replication and inhibition of host antiviral response by means of its host protein synthesis shutoff activity both in vitro and in vivo. We also demonstrated that, while the growth of Cal PA-XFS was attenuated in the lungs of infected animals, this mutant induced a stronger humoral response than Cal WT. Our findings clearly highlight the importance of PA-X in counteracting the host innate and acquired immune responses to influenza virus, an important global pathogen. This work demonstrates that inhibition of PA-X expression in influenza virus vaccine strains may provide a novel way of safely attenuating viral growth while inducing a more robust immune response.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
FIG 1
Rescue of Cal PA-XFS possessing mutations in the frameshift motif. (A) Schematic representation of Cal PA and PA-X proteins. Cal PA-X encodes a common N-terminal endonuclease domain (amino acid residues 1 to 191) of PA fused to a unique C-terminal region (41 amino acids) produced by a +1 reading frame of PA mRNA via ribosomal frameshifting. The frameshift motif is shown within a rectangle. (B) Reactivities of MAbs as determined by Western blotting. 293T cells were transfected with the indicated plasmids for 24 h. The cell lysates were subjected to Western blotting using the indicated MAb. pPA, pCAGGSCalPAflag; p134A, pCAGGSCalPA-X134Aflag; p134AΔC, pCAGGSCalPA-X134AΔCflag. (C) A549 cells infected with Cal WT or Cal PA-XFS or 293T cells transfected with pCAGGSCalPA-X134Aflag were labeled with 35S-labeled methionine and cysteine. The labeled cell lysates were used for immunoprecipitation (IP) by anti-PA-X or anti-PA MAb or for Western blotting (WB) using the indicated MAbs. The data shown are representative of two independent experiments.
FIG 2
Effects of PA-X expression on host protein synthesis and actin mRNA levels in human cells infected with Cal viruses. (A) A549 cells were either left uninfected or infected with Cal WT or Cal PA-XFS at an MOI of 1. At the indicated time points, cells were labeled with 35S-labeled methionine and cysteine for 30 min, and total lysates were resolved by SDS-PAGE. (B) Densitometric traces of labeled proteins in cells that were either left uninfected or infected with the viruses shown in panel A. (C and D) A549 or Calu-3 cells were either left uninfected or infected with Cal WT or Cal PA-XFS at an MOI of 3. At 4 or 12 hpi, total RNA was extracted from cell lysates and subjected to Northern blot analysis using a DIG-labeled antisense human β-actin probe. 18S and 28S rRNAs were stained with ethidium bromide and are shown as a loading control. The data shown are representative of two (A) or three (C and D) independent experiments.
FIG 3
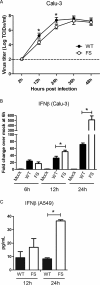
Effects of PA-X expression on viral growth and IFN-β expression in vitro. (A) Viral growth kinetics in Calu-3 cells. The cells were infected with either Cal WT or Cal PA-XFS at an MOI of 0.025. At the indicated time points, 10% of the supernatant was collected and titrated on MDCK cells. The dashed line indicates the detection limit of the assay. The data represent averages with standard deviations from two independent experiments with assays performed in triplicate (n = 6). (B) Calu-3 cells were either left uninfected or infected with Cal WT or Cal PA-XFS at an MOI of 3. At the indicated time points, total RNA was extracted and subjected to qRT-PCR analysis. The copy number in each sample was calculated using a standard curve of 10-fold serial dilutions of human IFN-β gene product. Fold change is the ratio of the copy number of each sample over that of mock-infected cells at 6 h. The mRNA levels represent averages with standard deviations. (C) A549 cells were either left uninfected or infected with Cal WT or Cal PA-XFS at an MOI of 10. IFN-β protein levels in culture supernatants at 12 or 24 hpi were determined by using a human IFN-β ELISA kit. The data represent averages with standard deviations. Asterisks indicate statistically significant differences by the Student t test (*, P < 0.05).
FIG 4
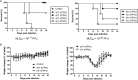
Survival and body weight change of mice infected with Cal viruses. (A and B) C57BL/6 mice were either mock infected or infected with Cal WT or Cal PA-XFS at a dose of 101, 102, 103, or 104 PFU intranasally. The survival (A) and body weight (B) of each mouse were monitored daily until 16 dpi (n = 5). Asterisks indicate statistically significant differences by the Student t test (*, P < 0.05).
FIG 5
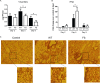
Viral growth, IFN-β mRNA expression, and pathology in lungs of the infected mice. C57BL/6 mice were mock infected or infected with Cal WT or Cal PA-XFS at a dose of 102 PFU intranasally. At 2, 5, or 8 dpi, four mice from each group were humanely sacrificed and the extracted whole lungs were homogenized in 1 ml of PBS. (A) Virus present in the homogenates was titrated on MDCK cells by plaque assay. (B) Total RNA was extracted from 100 μl of the homogenates, and IFN-β mRNA levels were determined by qRT-PCR analysis. Fold change is the ratio of the copy number of each sample over that of the control sample at 2 dpi. The data represent averages with standard deviations. Asterisks indicate statistically significant differences by the Student t test (*, P < 0.05). (C) Representative hematoxylin-and-eosin-stained images of uninfected or infected lungs at 8 dpi. Original magnification, ×10.
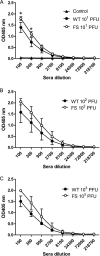
FIG 6
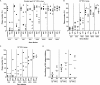
Titration of neutralization antibody in sera of mice infected with Cal viruses. (A to C) Neutralization assay was performed using serum samples collected at 16 dpi from surviving mice infected with Cal viruses at a dose of 101, 102, or 103 PFU. Each serum sample was serially diluted and mixed with 150 PFU of Cal WT, and then plaque numbers were counted. Data are presented as the percentages of the plaque number of control virus incubated with PBS. (D) Calculated serum dilutions showing 50% plaque inhibition. The data shown are averages with standard deviations of the results from 4 or 5 (control, 101, or 102 PFU virus) or 2 or 3 (103 PFU virus) individual mice. Asterisks indicate statistically significant differences by the Student t test (*, P < 0.05).
FIG 7
Titration of anti-HA antibodies in sera of mice infected with Cal viruses. Antibody titers were determined by ELISA on 96-well plate using Cal HA protein (0.1 μg/well) as an antigen. Serum samples were collected from surviving mice at 16 dpi. Data shown are the averages with standard deviations of the results from 4 or 5 (control, 101, or 102 PFU virus) or 2 or 3 (103 PFU virus) individual mice. Asterisks indicate statistically significant differences by the Student t test (*, P < 0.05).
Similar articles
- Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.
Nogales A, Martinez-Sobrido L, Topham DJ, DeDiego ML. Nogales A, et al. Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review. - Interplay of PA-X and NS1 Proteins in Replication and Pathogenesis of a Temperature-Sensitive 2009 Pandemic H1N1 Influenza A Virus.
Nogales A, Rodriguez L, DeDiego ML, Topham DJ, Martínez-Sobrido L. Nogales A, et al. J Virol. 2017 Aug 10;91(17):e00720-17. doi: 10.1128/JVI.00720-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28637750 Free PMC article. - Functional Evolution of the 2009 Pandemic H1N1 Influenza Virus NS1 and PA in Humans.
Nogales A, Martinez-Sobrido L, Chiem K, Topham DJ, DeDiego ML. Nogales A, et al. J Virol. 2018 Sep 12;92(19):e01206-18. doi: 10.1128/JVI.01206-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021892 Free PMC article. - Impacts of different expressions of PA-X protein on 2009 pandemic H1N1 virus replication, pathogenicity and host immune responses.
Lee J, Yu H, Li Y, Ma J, Lang Y, Duff M, Henningson J, Liu Q, Li Y, Nagy A, Bawa B, Li Z, Tong G, Richt JA, Ma W. Lee J, et al. Virology. 2017 Apr;504:25-35. doi: 10.1016/j.virol.2017.01.015. Epub 2017 Jan 29. Virology. 2017. PMID: 28142079 Free PMC article. - PA-X: a key regulator of influenza A virus pathogenicity and host immune responses.
Hu J, Ma C, Liu X. Hu J, et al. Med Microbiol Immunol. 2018 Nov;207(5-6):255-269. doi: 10.1007/s00430-018-0548-z. Epub 2018 Jul 4. Med Microbiol Immunol. 2018. PMID: 29974232 Free PMC article. Review.
Cited by
- Shutoff of Host Gene Expression in Influenza A Virus and Herpesviruses: Similar Mechanisms and Common Themes.
Rivas HG, Schmaling SK, Gaglia MM. Rivas HG, et al. Viruses. 2016 Apr 16;8(4):102. doi: 10.3390/v8040102. Viruses. 2016. PMID: 27092522 Free PMC article. Review. - Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.
Nogales A, Martinez-Sobrido L, Topham DJ, DeDiego ML. Nogales A, et al. Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review. - A Membrane-Anchored Short-Peptide Fusion Inhibitor Fully Protects Target Cells from Infections of Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus.
Tang X, Jin H, Chen Y, Li L, Zhu Y, Chong H, He Y. Tang X, et al. J Virol. 2019 Oct 29;93(22):e01177-19. doi: 10.1128/JVI.01177-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462566 Free PMC article. - An R195K Mutation in the PA-X Protein Increases the Virulence and Transmission of Influenza A Virus in Mammalian Hosts.
Sun Y, Hu Z, Zhang X, Chen M, Wang Z, Xu G, Bi Y, Tong Q, Wang M, Sun H, Pu J, Iqbal M, Liu J. Sun Y, et al. J Virol. 2020 May 18;94(11):e01817-19. doi: 10.1128/JVI.01817-19. Print 2020 May 18. J Virol. 2020. PMID: 32161172 Free PMC article. - The Role of Viral RNA Degrading Factors in Shutoff of Host Gene Expression.
Gaucherand L, Gaglia MM. Gaucherand L, et al. Annu Rev Virol. 2022 Sep 29;9(1):213-238. doi: 10.1146/annurev-virology-100120-012345. Epub 2022 Jun 7. Annu Rev Virol. 2022. PMID: 35671567 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources