Gamma-carboxylation and fragmentation of osteocalcin in human serum defined by mass spectrometry - PubMed (original) (raw)
Randomized Controlled Trial
. 2015 Jun;14(6):1546-55.
doi: 10.1074/mcp.M114.047621. Epub 2015 Apr 8.
Affiliations
- PMID: 25855755
- PMCID: PMC4458719
- DOI: 10.1074/mcp.M114.047621
Randomized Controlled Trial
Gamma-carboxylation and fragmentation of osteocalcin in human serum defined by mass spectrometry
Douglas S Rehder et al. Mol Cell Proteomics. 2015 Jun.
Abstract
Serum osteocalcin (Oc) concentration is a highly specific measure of bone turnover, but its circulating proteoform(s) have not been well defined. Based on immunological methods, the major forms are thought to be the intact polypeptide and a large N-terminal-mid molecule fragment for which there is no consensus on the precise sequence. Vitamin K-dependent gamma (γ)-carboxylated variants of Oc are also found in circulation but there have been no methods that can define how many of the three potential γ-carboxyglutamic acid (Gla) residues are γ-carboxylated or provide their relative abundances. Recent reports that uncarboxylated and partially γ-carboxylated Oc forms have hormonal function underscore the need for precise evaluation of Oc at all three potential γ-carboxylation sites. Herein, mass spectrometric immunoassay (MSIA) was used to provide qualitative and semiquantitative (relative percent abundance) information on Oc molecular variants as they exist in individual plasma and serum samples. Following verification that observable Oc proteoforms were accurately assigned and not simply ex vivo artifacts, MALDI-MSIA and ESI-MSIA were used to assess the relative abundance of Oc truncation and γ-carboxylation, respectively, in plasma from 130 patients enrolled in vitamin K supplementation trials. Human Oc was found to circulate in over a dozen truncated forms with each of these displaying anywhere from 0-3 Gla residues. The relative abundance of truncated forms was consistent and unaffected by vitamin K supplementation. In contrast, when compared with placebo, vitamin K supplementation dramatically increased the fractional abundance of Oc with three Gla residues, corresponding to a decrease in the fractional abundance of Oc with zero Gla residues. These findings unequivocally document that increased vitamin K intake reduces the uncarboxylated form of Oc. Several reports of a positive effect of vitamin K intake on insulin sensitivity in humans have shown that un- or undercarboxylation of Oc, unlike in mice, is not associated with insulin resistance. Analyses similar to those described here will be useful to understand the functional significance of Oc γ-carboxylation in human health and disease.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
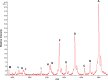
Fig. 1.
Oc from a single human blood plasma sample analyzed by MALDI-MSIA. The intact, full length protein is the most abundant form, but several previously undocumented N- and C-terminally truncated forms are also evident (Table I). Evidence described in the text indicates that these did not appear to form artifactually ex vivo. Because of prompt fragmentation induced by the laser (–23), γ-carboxyl post-translational modifications could not be observed by MALDI-MS. The spectrum shown is a single representative of spectra from 130 specimens analyzed (
supplemental Fig. S5
). Lower mass shoulder peaks as observed on peaks A, D, and F correspond to in-source neutral loss of water or ammonia. Higher mass shoulder peaks correspond to sodium adducts [M + Na]+.
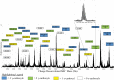
Fig. 2.
Carboxylated (COOH) and uncarboxylated (unCOOH) bovine Oc fortified into human serum and plasma at 120 ng/ml and incubated at 25 °C for up to 4 h followed by analysis by MALDI-MSIA. Cleavage of bovine Oc was minimal; the most striking change was a slight increase in L[2–49]V after 4 h incubation in serum or plasma as shown in the inset. The adjacent small peak at m/z 5620 is Y[1–48]P and did not increase in relative abundance over time. a) Pure COOH Oc standard, b) COOH Oc fortified into plasma then immediately extracted, c) COOH Oc fortified into plasma and incubated for 4 h, d) COOH Oc fortified into serum then immediately extracted, e) COOH Oc fortified into serum and incubated for 4 h, f) Pure unCOOH Oc standard, g) unCOOH Oc fortified into plasma then immediately extracted, h) unCOOH Oc fortified into plasma and incubated for 4 h, i) unCOOH Oc fortified into serum then immediately extracted, and j) unCOOH Oc fortified into serum and incubated for 4 h.
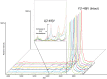
Fig. 3.
ESI-MSIA mass spectrum of Oc extracted from human blood plasma donated by a 24-yr old female. This sample was purchased from a commercial biobank for the purposes of initial assay development and was not part of Studies A-C described in the text. γ-carboxyl post-translational modifications were readily detected and mass mapped – revealing the precise number of γ-carboxyl groups present on each protein molecule. Nine differentially truncated forms of Oc were detected by ESI-MSIA (Table I). Magnesium and chromium adducts (which have previously been documented (39, 40) are likely derived from the metal electrospray needle. All mass spectral peak area integrals were calculated and used to determine the relative percent abundance of Oc with 0, 1, 2, and 3 γ-carboxyl groups. Peaks appear as solid black because protein ions are monoisotopically resolved (inset). * Indicates nonspecific detection of a multiply charged form of apolipoprotein A–I. Qualitatively, the lack of spacing between the isotopes indicates that the peak does not arise from Oc.
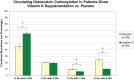
Fig. 4.
Detailed molecular comparison provided by ESI-MSIA of circulating Oc γ-carboxylation in volunteers receiving a vitamin K supplement or placebo (Study B). The relative percent abundance of circulating Oc molecules bearing 0, 1, 2, and 3 Gla residues is shown for each group. Samples were collected after 24 months of treatment. Error bars represent 95% confidence intervals. * Indicates statistical significance as determined by a t test (p < 0.001).
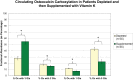
Fig. 5.
Circulating Oc γ-carboxylation in volunteers from Study C after vitamin K depletion (d13) and vitamin K supplementation (d41). The relative percent abundance of circulating Oc molecules bearing 0, 1, 2, and 3 Gla residues is shown for each group. Specimens analyzed were collected 7 days after initiation of each treatment. Error bars represent 95% confidence intervals. * Indicates statistical significance as determined by a paired t test (* for p < 0.001; † for p < 0.01).
Similar articles
- Shift of serum osteocalcin components between cord blood and blood at day 5 of life.
Shimizu N, Shima M, Hirai H, Nakajima S, Nishimura K, Yamaoka K, Okada S. Shimizu N, et al. Pediatr Res. 2002 Nov;52(5):656-9. doi: 10.1203/00006450-200211000-00009. Pediatr Res. 2002. PMID: 12409510 - Association of vitamin K status with adiponectin and body composition in healthy subjects: uncarboxylated osteocalcin is not associated with fat mass and body weight.
Knapen MH, Schurgers LJ, Shearer MJ, Newman P, Theuwissen E, Vermeer C. Knapen MH, et al. Br J Nutr. 2012 Sep 28;108(6):1017-24. doi: 10.1017/S000711451100626X. Epub 2011 Dec 5. Br J Nutr. 2012. PMID: 22136751 Clinical Trial. - Vitamin K-dependent carboxylation of osteocalcin affects the efficacy of teriparatide (PTH(1-34)) for skeletal repair.
Shimizu T, Takahata M, Kameda Y, Hamano H, Ito T, Kimura-Suda H, Todoh M, Tadano S, Iwasaki N. Shimizu T, et al. Bone. 2014 Jul;64:95-101. doi: 10.1016/j.bone.2014.04.005. Epub 2014 Apr 13. Bone. 2014. PMID: 24731926 - The role of vitamin K in soft-tissue calcification.
Theuwissen E, Smit E, Vermeer C. Theuwissen E, et al. Adv Nutr. 2012 Mar 1;3(2):166-73. doi: 10.3945/an.111.001628. Adv Nutr. 2012. PMID: 22516724 Free PMC article. Review. - Vitamin K and bone health in adult humans.
Bügel S. Bügel S. Vitam Horm. 2008;78:393-416. doi: 10.1016/S0083-6729(07)00016-7. Vitam Horm. 2008. PMID: 18374202 Review.
Cited by
- The effects of vitamin K-rich green leafy vegetables on bone metabolism: A 4-week randomised controlled trial in middle-aged and older individuals.
Sim M, Lewis JR, Prince RL, Levinger I, Brennan-Speranza TC, Palmer C, Bondonno CP, Bondonno NP, Devine A, Ward NC, Byrnes E, Schultz CJ, Woodman R, Croft K, Hodgson JM, Blekkenhorst LC. Sim M, et al. Bone Rep. 2020 Apr 26;12:100274. doi: 10.1016/j.bonr.2020.100274. eCollection 2020 Jun. Bone Rep. 2020. PMID: 32455149 Free PMC article. - Twelve weeks of a diet and exercise intervention alters the acute bone response to exercise in adolescent females with overweight/obesity.
Kurgan N, Skelly LE, Ludwa IA, Klentrou P, Josse AR. Kurgan N, et al. Front Physiol. 2023 Jan 4;13:1049604. doi: 10.3389/fphys.2022.1049604. eCollection 2022. Front Physiol. 2023. PMID: 36685198 Free PMC article. - Serum Osteocalcin and Testosterone Concentrations in Adult Males with or without Primary Osteoporosis: A Meta-Analysis.
Liu ZY, Yang Y, Wen CY, Rong LM. Liu ZY, et al. Biomed Res Int. 2017;2017:9892048. doi: 10.1155/2017/9892048. Epub 2017 Aug 2. Biomed Res Int. 2017. PMID: 28831400 Free PMC article. - Characterization of Gla proteoforms and non-Gla peptides of gamma carboxylated proteins: Application to quantification of prothrombin proteoforms in human plasma.
Singh DK, Basit A, Rettie AE, Alade N, Thummel K, Prasad B. Singh DK, et al. Anal Chim Acta. 2023 Dec 15;1284:341972. doi: 10.1016/j.aca.2023.341972. Epub 2023 Nov 5. Anal Chim Acta. 2023. PMID: 37996163 Free PMC article. - Recombinant Antibodies with Unique Specificities Allow for Sensitive and Specific Detection of Uncarboxylated Osteocalcin in Human Circulation.
Arponen M, Brockmann EC, Kiviranta R, Lamminmäki U, Ivaska KK. Arponen M, et al. Calcif Tissue Int. 2020 Dec;107(6):529-542. doi: 10.1007/s00223-020-00746-8. Epub 2020 Aug 24. Calcif Tissue Int. 2020. PMID: 32839842 Free PMC article.
References
- Hauschka P. V., Lian J. B., Cole D. E., Gundberg C. M. (1989) Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol. Rev. 69, 990–1047 - PubMed
- Eastell R., Hannon R. A. (2008) Biomarkers of bone health and osteoporosis risk. Proc. Nutr. Soc. 67, 157–162 - PubMed
- Garnero P., Grimaux M., Demiaux B., Preaudat C., Seguin P., Delmas P. D. (1992) Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J. Bone Miner Res. 7, 1389–1398 - PubMed
- Booth S. L., Martini L., Peterson J. W., Saltzman E., Dallal G. E., Wood R. J. (2003) Dietary phylloquinone depletion and repletion in older women. J. Nutr. 133, 2565–2569 - PubMed
- Booth S. L., Al Rajabi A. (2008) Determinants of vitamin K status in humans. Vitam. Horm. 78, 1–22 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK082542/DK/NIDDK NIH HHS/United States
- DK082542/DK/NIDDK NIH HHS/United States
- DK090958/DK/NIDDK NIH HHS/United States
- R01 AR038460/AR/NIAMS NIH HHS/United States
- AG14759/AG/NIA NIH HHS/United States
- DK69341/DK/NIDDK NIH HHS/United States
- R01 DK069341/DK/NIDDK NIH HHS/United States
- AR38460/AR/NIAMS NIH HHS/United States
- R01 AG019147/AG/NIA NIH HHS/United States
- R24 DK090958/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources