UPR(mt)-mediated cytoprotection and organismal aging - PubMed (original) (raw)
Review
UPR(mt)-mediated cytoprotection and organismal aging
Anna M Schulz et al. Biochim Biophys Acta. 2015 Nov.
Abstract
Time- or age-dependent accumulation of mitochondrial damage and dysfunction is strongly associated with aging [1]. Thus, a major biomedical goal is to identify and therapeutically manipulate those inherent programs that protect against mitochondrial dysfunction to promote cell survival and organismal health. The mitochondrial unfolded protein response (UPR(mt)) is such a protective transcriptional response mediated by mitochondrial-to-nuclear signaling that includes mitochondrial proteostasis genes to stabilize mitochondrial function, metabolic adaptations, as well as an innate immunity program. Here, we review the UPR(mt) and its role during a variety of forms of mitochondrial dysfunction including those caused by mutations in respiratory chain genes as well as upon exposure to pathogens that produce mitochondrial toxins. We also review recent data in support of and against the emerging role of the UPR(mt) during aging and longevity. This article is part of a Special Issue entitled: Mitochondrial Dysfunction in Aging.
Keywords: ATFS-1; Innate immunity; Metabolism; Mitochondrial dysfunction; Mitochondrial unfolded protein response.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures
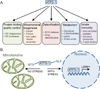
Figure 1. UPRmt activation via ATFS-1 regulates a broad transcriptional program
(A) In response to mitochondrial damage or stress ATFS-1 induces genes involved in mitochondrial repair mechanisms including protein folding and protein quality control, as well as those involved in mitochondrial biogenesis, the detoxification response, metabolism and innate immune gene transcription. (B) The UPRmt is activated during conditions such as mtDNA depletion, respiratory chain dysfunction, increased ROS or increased mitochondrial unfolded proteins, which is regulated by the mitochondrial protein-import efficiency of the transcription factor ATFS-1. In the absence of stress, ATFS-1 localizes to mitochondria via its mitochondria targeting signal (MTS), where it is degraded by the protease Lon. However, during mitochondrial dysfunction or stress, general mitochondrial protein import is attenuated, leading to the accumulation of a portion of ATFS-1 in the cytosol, followed by its translocation to the nucleus via its nuclear localization signal (NLS). In the nucleus, ATFS-1 induces the mitochondrial protective genes described in Figure 1A, which promote survival and recovery from mitochondrial stress.
Figure 2. GCN2 phosphorylates eIF2α during mitochondrial dysfunction
Dysfunctional mitochondria are major sources of reactive oxygen species (ROS), which accumulate both inside mitochondria and in the cytosol. ROS as well as imbalanced amino acid levels activate the kinase GCN2, which phosphorylates eIF2α to attenuate global mRNA translation. Reduced protein synthesis during mitochondrial stress reduces the burden of unfolded proteins on mitochondria, which facilitates overall recovery. In addition to reducing global protein synthesis, eIF2α phosphorylation selectively increases the translation of mRNAs containing uORFs such as ATF4, which induces transcription of the gene encoding CHOP. Several atfs-1 mRNAs contain uORFs suggesting that it may also be preferentially translated during eIF2α phosphorylation.
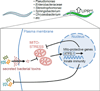
Figure 3. UPRmt-mediated innate immunity in response to bacterial infection
In their natural habitat worms are exposed to a variety of bacterial species, many of which cause UPRmt activation [48]. Secreted bacterial toxins such as _Pseudomonas aeruginosa_-produced cyanide target mitochondria and activate the UPRmt. Cyanide impairs the respiratory chain, thereby perturbing mitochondrial protein import causing ATFS-1 to traffic to the nucleus. In the nucleus, ATFS-1 induces mitochondrial protective genes such as mitochondrial chaperones and proteases, but also innate immune genes such as antimicrobial peptides and secreted lysozymes [73]. Worms with an activated UPRmt have reduced intestinal accumulation of P. aeruginosa and survive longer when exposed to the pathogen [73] indicating that the UPRmt promotes an innate immune response that confers resistance to pathogenic bacteria.
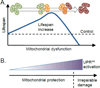
Figure 4. Mitochondrial stress and lifespan regulation
(A) Lifespan increases with moderate levels of mitochondrial dysfunction and is reduced when the damage is too severe. During mild mitochondrial dysfunction (orange), which may affect the entire mitochondrial pool or individuals organelles, the organism is able to promote mitochondrial recovery through physiological alterations that positively affect lifespan. These adaptations include pro-survival metabolic alterations, maintenance of the mitochondrial protein folding environment and resistance to pathogens. (B) The UPRmt is activated in response to mitochondrial dysfunction and promotes mitochondrial repair and metabolic adaptations. At some point, the mitochondrial damage becomes irreparable offsetting the UPRmt-mediated protective effects (dashed line). During severe mitochondrial dysfunction UPRmt activation still occurs, but may not be sufficient to maintain mitochondrial homeostasis and protect survival.
Similar articles
- Metabolism and the UPR(mt).
Lin YF, Haynes CM. Lin YF, et al. Mol Cell. 2016 Mar 3;61(5):677-682. doi: 10.1016/j.molcel.2016.02.004. Mol Cell. 2016. PMID: 26942672 Free PMC article. Review. - Dysregulation of the Mitochondrial Unfolded Protein Response Induces Non-Apoptotic Dopaminergic Neurodegeneration in C. elegans Models of Parkinson's Disease.
Martinez BA, Petersen DA, Gaeta AL, Stanley SP, Caldwell GA, Caldwell KA. Martinez BA, et al. J Neurosci. 2017 Nov 15;37(46):11085-11100. doi: 10.1523/JNEUROSCI.1294-17.2017. Epub 2017 Oct 13. J Neurosci. 2017. PMID: 29030433 Free PMC article. - Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection.
Pellegrino MW, Nargund AM, Kirienko NV, Gillis R, Fiorese CJ, Haynes CM. Pellegrino MW, et al. Nature. 2014 Dec 18;516(7531):414-7. doi: 10.1038/nature13818. Epub 2014 Sep 28. Nature. 2014. PMID: 25274306 Free PMC article. - Dietary cobalt oxide nanoparticles alleviate aging through activation of mitochondrial UPR in Caenorhabditis elegans.
Cong W, Wang Y, Yuan C, Xu M, Wang H, Hu Y, Dai X, Weng Y, Timashev P, Liang XJ, Huang Y. Cong W, et al. Theranostics. 2023 May 27;13(10):3276-3289. doi: 10.7150/thno.81817. eCollection 2023. Theranostics. 2023. PMID: 37351160 Free PMC article. - Mitochondrial unfolded protein response: An emerging pathway in human diseases.
Zhu L, Zhou Q, He L, Chen L. Zhu L, et al. Free Radic Biol Med. 2021 Feb 1;163:125-134. doi: 10.1016/j.freeradbiomed.2020.12.013. Epub 2020 Dec 21. Free Radic Biol Med. 2021. PMID: 33347985 Review.
Cited by
- The Aging Epigenome.
Booth LN, Brunet A. Booth LN, et al. Mol Cell. 2016 Jun 2;62(5):728-44. doi: 10.1016/j.molcel.2016.05.013. Mol Cell. 2016. PMID: 27259204 Free PMC article. Review. - Cell-Nonautonomous Regulation of Proteostasis in Aging and Disease.
Morimoto RI. Morimoto RI. Cold Spring Harb Perspect Biol. 2020 Apr 1;12(4):a034074. doi: 10.1101/cshperspect.a034074. Cold Spring Harb Perspect Biol. 2020. PMID: 30962274 Free PMC article. Review. - A multimethod computational simulation approach for investigating mitochondrial dynamics and dysfunction in degenerative aging.
Hoffman TE, Barnett KJ, Wallis L, Hanneman WH. Hoffman TE, et al. Aging Cell. 2017 Dec;16(6):1244-1255. doi: 10.1111/acel.12644. Epub 2017 Aug 16. Aging Cell. 2017. PMID: 28815872 Free PMC article. - Qualitative and Quantitative Ovarian and Peripheral Blood Mitochondrial DNA (mtDNA) Alterations: Mechanisms and Implications for Female Fertility.
Busnelli A, Navarra A, Levi-Setti PE. Busnelli A, et al. Antioxidants (Basel). 2021 Jan 5;10(1):55. doi: 10.3390/antiox10010055. Antioxidants (Basel). 2021. PMID: 33466415 Free PMC article. Review. - Inhibition of High-Temperature Requirement Protein A2 Protease Activity Represses Myogenic Differentiation via UPRmt.
Sun H, Shen L, Zhang P, Lin F, Ma J, Wu Y, Yu H, Sun L. Sun H, et al. Int J Mol Sci. 2022 Oct 4;23(19):11761. doi: 10.3390/ijms231911761. Int J Mol Sci. 2022. PMID: 36233059 Free PMC article.
References
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. 1966. Biochimica et biophysica acta. 2011;1807:1507–1538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical