All-atom 3D structure prediction of transmembrane β-barrel proteins from sequences - PubMed (original) (raw)
All-atom 3D structure prediction of transmembrane β-barrel proteins from sequences
Sikander Hayat et al. Proc Natl Acad Sci U S A. 2015.
Abstract
Transmembrane β-barrels (TMBs) carry out major functions in substrate transport and protein biogenesis but experimental determination of their 3D structure is challenging. Encouraged by successful de novo 3D structure prediction of globular and α-helical membrane proteins from sequence alignments alone, we developed an approach to predict the 3D structure of TMBs. The approach combines the maximum-entropy evolutionary coupling method for predicting residue contacts (EVfold) with a machine-learning approach (boctopus2) for predicting β-strands in the barrel. In a blinded test for 19 TMB proteins of known structure that have a sufficient number of diverse homologous sequences available, this combined method (EVfold_bb) predicts hydrogen-bonded residue pairs between adjacent β-strands at an accuracy of ∼70%. This accuracy is sufficient for the generation of all-atom 3D models. In the transmembrane barrel region, the average 3D structure accuracy [template-modeling (TM) score] of top-ranked models is 0.54 (ranging from 0.36 to 0.85), with a higher (44%) number of residue pairs in correct strand-strand registration than in earlier methods (18%). Although the nonbarrel regions are predicted less accurately overall, the evolutionary couplings identify some highly constrained loop residues and, for FecA protein, the barrel including the structure of a plug domain can be accurately modeled (TM score = 0.68). Lower prediction accuracy tends to be associated with insufficient sequence information and we therefore expect increasing numbers of β-barrel families to become accessible to accurate 3D structure prediction as the number of available sequences increases.
Keywords: de novo 3D structure prediction; evolutionary couplings; hydrogen bonding; maximum-entropy analysis; transmembrane β-barrels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
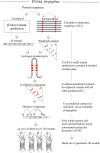
Fig. 1.
EVfold_bb pipeline to de novo fold transmembrane β-barrels. EVFold-PLM is used to generate evolutionary couplings (ECs) from a multiple-sequence alignment of the target protein. Boctopus2 is used to assign β-strands. Alternative strand registrations are compared for successive relative shifts of adjacent strands up to plus or minus three residues. The configuration with the largest sum of EC values is chosen and distance constraints are applied on N–O atoms of alternate residue pairs. In addition, other nonstrand–strand constraints are used to de novo fold the protein. Multiple models are generated and blindly ranked.
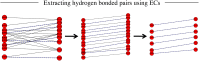
Fig. 2.
Evolutionary couplings give residue pairs that are hydrogen bonded. From the evolutionary couplings (ECs) (black) predicted between residues (red) on strands 5 and 6 of EstA (Left), a subset of hydrogen-bonded pairs (dashed lines, where N–O distance ≤ 3.4 Å) is extracted. Predicted adjacent β-strands are shifted plus or minus three residues relative to each other to generate alternate configurations of residue pairs (Center). To select the best configuration, the EC strength of all pairs is summed and the highest-scoring configuration is selected; then distance constraints are applied to N–O atoms of alternate residue pairs (Right) to reflect N–H ... O = C hydrogen bonds.
Fig. 3.
Blinded benchmark de novo 3D models of transmembrane β-barrels. Shown are predicted contact maps (red, ECs; gray, crystal contacts ≤ 5 Å; blue, gaps in crystal structure) and front and top views of folded structures (red, de novo folded; gray, crystal structure) for six proteins in the dataset.
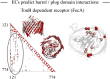
Fig. 4.
ECs predict interactions between loops/plugs and the barrel domain. Interactions between the barrel (245–774) and the plug (121–244) domain in FecA are highlighted in the predicted contact map (red, ECs; gray, crystal contacts ≤ 5 Å). Top 10 interdomain contacts between the barrel (red) and the plug domain (pink) are shown on the crystal structure and have a PPV of 0.9.
Similar articles
- Predicting transmembrane beta-barrels and interstrand residue interactions from sequence.
Waldispühl J, Berger B, Clote P, Steyaert JM. Waldispühl J, et al. Proteins. 2006 Oct 1;65(1):61-74. doi: 10.1002/prot.21046. Proteins. 2006. PMID: 16858668 - Molecular mechanism of ferricsiderophore passage through the outer membrane receptor proteins of Escherichia coli.
Chakraborty R, Storey E, van der Helm D. Chakraborty R, et al. Biometals. 2007 Jun;20(3-4):263-74. doi: 10.1007/s10534-006-9060-9. Epub 2006 Dec 22. Biometals. 2007. PMID: 17186377 Review. - TMB finding pipeline: novel approach for detecting beta-barrel membrane proteins in genomic sequences.
Gromiha MM, Yabuki Y, Suwa M. Gromiha MM, et al. J Chem Inf Model. 2007 Nov-Dec;47(6):2456-61. doi: 10.1021/ci700222s. Epub 2007 Oct 24. J Chem Inf Model. 2007. PMID: 17958348 - Evaluation of methods for predicting the topology of beta-barrel outer membrane proteins and a consensus prediction method.
Bagos PG, Liakopoulos TD, Hamodrakas SJ. Bagos PG, et al. BMC Bioinformatics. 2005 Jan 12;6:7. doi: 10.1186/1471-2105-6-7. BMC Bioinformatics. 2005. PMID: 15647112 Free PMC article. - Redefining the goals of protein secondary structure prediction.
Rost B, Sander C, Schneider R. Rost B, et al. J Mol Biol. 1994 Jan 7;235(1):13-26. doi: 10.1016/s0022-2836(05)80007-5. J Mol Biol. 1994. PMID: 8289237 Review.
Cited by
- Pan-cancer structurome reveals overrepresentation of beta sandwiches and underrepresentation of alpha helical domains.
Medvedev KE, Schaeffer RD, Chen KS, Grishin NV. Medvedev KE, et al. Sci Rep. 2023 Jul 25;13(1):11988. doi: 10.1038/s41598-023-39273-5. Sci Rep. 2023. PMID: 37491511 Free PMC article. - Exploring microbial functional biodiversity at the protein family level-From metagenomic sequence reads to annotated protein clusters.
Baltoumas FA, Karatzas E, Paez-Espino D, Venetsianou NK, Aplakidou E, Oulas A, Finn RD, Ovchinnikov S, Pafilis E, Kyrpides NC, Pavlopoulos GA. Baltoumas FA, et al. Front Bioinform. 2023 Mar 3;3:1157956. doi: 10.3389/fbinf.2023.1157956. eCollection 2023. Front Bioinform. 2023. PMID: 36959975 Free PMC article. Review. - Machine learning in computational modelling of membrane protein sequences and structures: From methodologies to applications.
Sun J, Kulandaisamy A, Liu J, Hu K, Gromiha MM, Zhang Y. Sun J, et al. Comput Struct Biotechnol J. 2023 Jan 28;21:1205-1226. doi: 10.1016/j.csbj.2023.01.036. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 36817959 Free PMC article. Review. - Improved Protein Structure Prediction Using a New Multi-Scale Network and Homologous Templates.
Su H, Wang W, Du Z, Peng Z, Gao SH, Cheng MM, Yang J. Su H, et al. Adv Sci (Weinh). 2021 Dec;8(24):e2102592. doi: 10.1002/advs.202102592. Epub 2021 Oct 31. Adv Sci (Weinh). 2021. PMID: 34719864 Free PMC article. - Computational structure prediction provides a plausible mechanism for electron transfer by the outer membrane protein Cyc2 from Acidithiobacillus ferrooxidans.
Jiang V, Khare SD, Banta S. Jiang V, et al. Protein Sci. 2021 Aug;30(8):1640-1652. doi: 10.1002/pro.4106. Epub 2021 May 25. Protein Sci. 2021. PMID: 33969560 Free PMC article.
References
- Schleiff E, Becker T. Common ground for protein translocation: Access control for mitochondria and chloroplasts. Nat Rev Mol Cell Biol. 2011;12(1):48–59. - PubMed
- Pagès JM, James CE, Winterhalter M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol. 2008;6(12):893–903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources