Diacylglycerol Metabolism and Signaling Is a Driving Force Underlying FASN Inhibitor Sensitivity in Cancer Cells - PubMed (original) (raw)
Diacylglycerol Metabolism and Signaling Is a Driving Force Underlying FASN Inhibitor Sensitivity in Cancer Cells
Daniel I Benjamin et al. ACS Chem Biol. 2015.
Abstract
Fatty acid synthase (FASN) generates the de novo source of lipids for cell proliferation and is a promising cancer therapy target. Development of FASN inhibitors, however, necessitates a better understanding of sensitive and resistant cancer types to optimize patient treatment. Indeed, testing the cytotoxic effects of FASN inhibition across human cancer cells revealed diverse sensitivities. We show here that metabolic incorporation of glucose into specific complex lipid species strongly predicts FASN inhibitor sensitivity. We also show that the levels of one of these lipid classes, protein kinase C (PKC) stimulator diacylglycerols, are lowered upon FASN inhibitor treatment in sensitive compared to resistant cells and that PKC activators and inhibitors rescue cell death in sensitive cells and sensitize resistant cells, respectively. Our findings not only reveal a biomarker for predicting FASN sensitivity in cancer cells but also a put forth a heretofore unrecognized mechanism underlying the anticancer effects of FASN inhibitors.
Figures
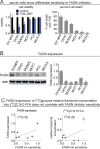
Figure 1. Cancer cells show differential sensitivity to FASN inhibitor TVB-3567
(A) Cancer cell viability in response to treatment with vehicle DMSO or FASN inhibitor TVB-3567 (1 μM) was measured across a panel of human cancer cells (231 MFP and MCF7 breast cancer, C8161 and MUM2C melanoma, PC3 prostate cancer, and SKOV3 ovarian cancer). Cells were treated with either vehicle DMSO or TVB3567 in 1% serum-containing media and viability was measured 7 d after treatment by either WST-1 assay or Hoescht staining cell viability assays. (B) FASN expression across the six cancer cell lines, determined by Western blotting and quantified by densitometry across. (C) Cancer cell sensitivity to TVB-3567 is poorly correlated with FASN expression (Pearson correlation coefficient of r2= 0.32) and with relative fractional [13C] glucose incorporation into palmitate over 24 h (Pearson correlation coefficient of r2 = 0.26). For FASN inhibitor sensitivity in (C), we have taken the percent cell death data from (A) and normalized the data against 231MFP sensitivity (set to 1). Relative fractional incorporation of glucose into palmitate was measured by SRM-based LC-MS/MS. Values are expressed as mean ± sem; n=3–5 per group. Significance is expressed in (A) as *p<0.05 compared to control.
Figure 2. Mapping [13C]glucose relative fractional incorporation into palmitate-containing complex lipid species
(A) Biochemical pathway map describing full [13C]glucose incorporation into complex lipid species through labeling of [13C]glycerol-3-phosphate backbone and [13C]palmitate of complex lipids. (B) Relative fractional [13C]glucose incorporation into fully labeled [13C]palmitate and palmitate containing complex lipid species. The relative fractional incorporation of isotopic glucose into each complex lipid species was calculated by dividing the levels of full isotopic incorporation into the particular lipid species in 24 h in the [13C]glucose-treated cells by the steady-state levels of the corresponding [12C]lipid species in the [12C]glucose-treated cells in 24 h, and then normalized to the fractional incorporation in 231MFP cells. This relative fractional incorporation is displayed as both a heat map as well as individual bar graphs for each lipid species. Dark and light blue on the heatmap denote high and low fractional incorporation, respectively. Data are presented as mean ± sem; n=4 per group.
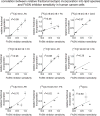
Figure 3. Correlation between relative fractional incorporation of isotopic glucose into complex lipid species and FASN inhibitor sensitivity reveals predictive biomarkers
We show correlation plots between FASN inhibitor sensitivity and fractional isotopic incorporation of [13C]glucose into fully labeled complex lipids. We find that [13C] C16:0 LPA, C16:0/18:1 DAG, C16:0/18:1 PC, and C16:0 LPC (all m+19), but not other lipid species, strongly correlate with TVB-3567 sensitivity across the six cancer cell lines tested in Fig. 1 (Pearson correlation coefficients of r2>0.97). Data are presented as mean ± sem; n=4 per group.
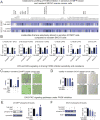
Figure 4. Metabolomic profiling of FASN inhibition reveals diacylglycerol metabolism and signaling as important drivers of FASN inhibitor sensitivity
(A) We performed an SRM-based LC-MS/MS steady-state metabolomic analysis of 154 lipid species to identify metabolomics alterations upon FASN inhibition in the sensitive 231MFP and resistant SKOV3 cells. 231MFP and SKOV3 cells were treated with vehicle (DMSO, 0.1 %) or TVB-3567 (1 μM) for 24 h in serum-free media after which cellular lipidomes were subjected to metabolomic analysis. The heat map shows the relative levels of all lipids measured in this experiment normalized to their respective controls. Darker and lighter blue shading on the heat map corresponds to higher and lower relative metabolite levels compared with DMSO treated controls, respectively. (B) We wanted to identify metabolites that were significantly changing in the sensitive 231MFP cells that were altered less or were not changing in the resistant SKOV3 cells, as these lipid species may represent drivers of FASN inhibitor sensitivity. Shown are the relative levels of metabolites that were significantly (p<0.05) and robustly (> 3-fold) altered in 231MFP cells and not in SKOV3 cells compared with respective vehicle-treated controls. Relative levels are normalized to the controls of each respective cell line. FASN inhibition with TVB-3567 significantly and robustly lowers levels of DAGs and LPA/LPA-ether (LPAe) species in 231MFP cells but not in SKOV3 cells. (C) The cell viability impairments conferred by TVB-3567 treatment in 231MFP cells (1 μM) was significantly rescued by the addition of the DAG-independent PKC activator PMA (1 μM) but not by LPA (200 nM). (D) Treatment of SKOV3 cells with the PKC inhibitor Go 6983 (1 μM) hypersensitizes them to FASN inhibition with TVB-3567. Pharmacological agents in (C) and (D) were co-treated in 231MFP cells for 4 d in serum-free media and cell viability was measured using the Hoescht stain cell viability assay. (E, F) Levels of phosphorylated ribosomal protein S6 upon treatment of 231MFP or SKOV3 cells with vehicle (DMSO), TVB-3567 (1 μM), PMA (1 μM), and/or Go 6983 (1 μM) for 3 d in serum-free media. Phospho-S6 was quantified in relation to expression of loading controls cyclophilin and RAB11 for 231MFP and SKOV3, respectively. Data is presented as mean ± sem; n=4–5 per group. Significance is presented in (B–F) as *p<0.05 compared with vehicle-treated control and # p<0.05 between 231 MFP and SKOV3 TVB-3567 treated groups.
References
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews. Cancer. 2007;7:763–777. - PubMed
- Oslob JD, Johnson RJ, Cai H, Feng SQ, Hu L, Kosaka Y, Lai J, Sivaraja M, Tep S, Yang H, Zaharia CA, Evanchik MJ, McDowell RS. Imidazopyridine-Based Fatty Acid Synthase Inhibitors That Show Anti-HCV Activity and in Vivo Target Modulation. ACS medicinal chemistry letters. 2013;4:113–117. - PMC - PubMed
- Pandey PR, Liu W, Xing F, Fukuda K, Watabe K. Anti-cancer drugs targeting fatty acid synthase (FAS) Recent patents on anti-cancer drug discovery. 2012;7:185–197. - PubMed
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nature reviews. Cancer. 2007;7:281–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous