Increased prevalence of EPAS1 variant in cattle with high-altitude pulmonary hypertension - PubMed (original) (raw)
Timothy N Holt 2, Joy D Cogan 3, Bethany Womack 3, John A Phillips 3rd 3, Chun Li 4, Zachary Kendall 3, Kurt R Stenmark 5, Milton G Thomas 6, R Dale Brown 5, Suzette R Riddle 5, James D West 1, Rizwan Hamid 3
Affiliations
- PMID: 25873470
- PMCID: PMC4399003
- DOI: 10.1038/ncomms7863
Increased prevalence of EPAS1 variant in cattle with high-altitude pulmonary hypertension
John H Newman et al. Nat Commun. 2015.
Abstract
High-altitude pulmonary hypertension (HAPH) has heritable features and is a major cause of death in cattle in the Rocky Mountains, USA. Although multiple genes are likely involved in the genesis of HAPH, to date no major gene variant has been identified. Using whole-exome sequencing, we report the high association of an EPAS1 (HIF2α) double variant in the oxygen degradation domain of EPAS1 in Angus cattle with HAPH, mean pulmonary artery pressure >50 mm Hg in two independent herds. Expression analysis shows upregulation of 26 of 27 HIF2α target genes in EPAS1 carriers with HAPH. Of interest, this variant appears to be prevalent in lowland cattle, in which 41% of a herd of 32 are carriers, but the variant may only have a phenotype when the animal is hypoxemic at altitude. The EPAS1 variant will be a tool to determine the cells and signalling pathways leading to HAPH.
Figures
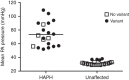
Figure 1. Mean pulmonary arterial pressure in 41 Angus cattle dwelling at high altitude.
Of the 20 HAPH (high-altitude pulmonary hypertension PAP>50 mm Hg), 15 carried the EPAS1 variant and all of the cattle with the highest PAP carried the variant. Four of the 21 unaffected cattle carried the variant, and these 4 had the highest pressure, albeit normal PAP <38 mm Hg of the group.
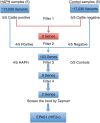
Figure 2. Sequence of filtering and analysis of WES data using an autosomal dominant model.
First filter was variant found in all five HAPH and in none unaffected. Second filter was the presence in 4/5 HAPH and 1/5 affected, yielding 103 genes. Filter 3 was 4/5 HAPH and 0/5 unaffected, yielding 9 genes. Sanger sequencing reduced the number to two candidates, EPAS1 and PDPR. Taqman assay revealed no association with PDPR, but highly significant association of HAPH with EPAS1 variant.
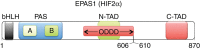
Figure 3. Graphic depiction of the known EPAS1 domains.
bHLH, basic Helix–Loop–Helix; PAS, Per-Arnt-Sim domain; ODDD, oxygen-dependent degradation domain; N-TAD, N-terminal transactivation domain; C-TAD, C-terminal transactivation domain. The two variants are in the ODDD domain of the protein.
Similar articles
- Evaluation of EPAS1 variants for association with bovine congestive heart failure.
Heaton MP, Bassett AS, Whitman KJ, Krafsur GM, Lee SI, Carlson JM, Clark HJ, Smith HR, Pelster MC, Basnayake V, Grotelueschen DM, Vander Ley BL. Heaton MP, et al. F1000Res. 2019 Jul 25;8:1189. doi: 10.12688/f1000research.19951.1. eCollection 2019. F1000Res. 2019. PMID: 31543958 Free PMC article. - Pulmonary arterial pressure testing for high mountain disease in cattle.
Holt TN, Callan RJ. Holt TN, et al. Vet Clin North Am Food Anim Pract. 2007 Nov;23(3):575-96, vii. doi: 10.1016/j.cvfa.2007.08.001. Vet Clin North Am Food Anim Pract. 2007. PMID: 17920462 Review. - The genetic factor influencing pulmonary hypertension in cattle at high altitude.
Weir EK, Tucker A, Reeves JT, Will DH, Grover RF. Weir EK, et al. Cardiovasc Res. 1974 Nov;8(6):745-9. doi: 10.1093/cvr/8.6.745. Cardiovasc Res. 1974. PMID: 4457227 No abstract available. - EPAS1 and EGLN1 associations with high altitude sickness in Han and Tibetan Chinese at the Qinghai-Tibetan Plateau.
Buroker NE, Ning XH, Zhou ZN, Li K, Cen WJ, Wu XF, Zhu WZ, Scott CR, Chen SH. Buroker NE, et al. Blood Cells Mol Dis. 2012 Aug 15;49(2):67-73. doi: 10.1016/j.bcmd.2012.04.004. Epub 2012 May 15. Blood Cells Mol Dis. 2012. PMID: 22595196 - Gene expression in chronic high altitude diseases.
León-Velarde F, Mejía O. León-Velarde F, et al. High Alt Med Biol. 2008 Summer;9(2):130-9. doi: 10.1089/ham.2007.1077. High Alt Med Biol. 2008. PMID: 18578644 Review.
Cited by
- Hypoxia-Inducible Factor Activators in Renal Anemia: Current Clinical Experience.
Sanghani NS, Haase VH. Sanghani NS, et al. Adv Chronic Kidney Dis. 2019 Jul;26(4):253-266. doi: 10.1053/j.ackd.2019.04.004. Adv Chronic Kidney Dis. 2019. PMID: 31477256 Free PMC article. Review. - Evaluation of EPAS1 variants for association with bovine congestive heart failure.
Heaton MP, Bassett AS, Whitman KJ, Krafsur GM, Lee SI, Carlson JM, Clark HJ, Smith HR, Pelster MC, Basnayake V, Grotelueschen DM, Vander Ley BL. Heaton MP, et al. F1000Res. 2019 Jul 25;8:1189. doi: 10.12688/f1000research.19951.1. eCollection 2019. F1000Res. 2019. PMID: 31543958 Free PMC article. - Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains.
Culley MK, Chan SY. Culley MK, et al. J Clin Invest. 2018 Aug 31;128(9):3704-3715. doi: 10.1172/JCI120847. Epub 2018 Aug 6. J Clin Invest. 2018. PMID: 30080181 Free PMC article. Review. - Global dispersal and adaptive evolution of domestic cattle: a genomic perspective.
Xia X, Qu K, Wang Y, Sinding MS, Wang F, Hanif Q, Ahmed Z, Lenstra JA, Han J, Lei C, Chen N. Xia X, et al. Stress Biol. 2023 Apr 18;3(1):8. doi: 10.1007/s44154-023-00085-2. Stress Biol. 2023. PMID: 37676580 Free PMC article. Review. - Varied hypoxia adaptation patterns of embryonic brain at different development stages between Tibetan and Dwarf laying chickens.
Tang Q, Yu R, Wang Y, Xie F, Zhang H, Wu C, Fang M. Tang Q, et al. BMC Genomics. 2023 Jun 21;24(1):342. doi: 10.1186/s12864-023-09457-4. BMC Genomics. 2023. PMID: 37344809 Free PMC article.
References
- Glover G. H. & Newsom I. E. Brisket disease (dropsy of high altitude). Colorado Agricultural Experiment Station 204 Preliminary Report 3–24 (1915).
- Rhodes J. Comparative physiology of hypoxic pulmonary hypertension: historical clues from Brisket disease. J. Appl. Physiol. 98, 1092–1100 (2005). - PubMed
- Grover R. F., Reeves J. T., Will D. H. & Blount S. G. Jr Pulmonary vasoconstriction in steers at high altitude. J. Appl. Physiol. 18, 567–574 (1963). - PubMed
- Grover R. F. Pulmonary circulation in animals and man at high altitude. Ann. N.Y. Acad. Sci. 27, 632–639 (1965). - PubMed
- Holt T. N. & Callan R. J. Pulmonary arterial pressure testing for high mountain disease in cattle. Vet. Clin. Food Anim. 23, 575–596 (2007). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01HL102020-01/HL/NHLBI NIH HHS/United States
- R01 HL102020/HL/NHLBI NIH HHS/United States
- R01 HL125827/HL/NHLBI NIH HHS/United States
- U01 HG007674/HG/NHGRI NIH HHS/United States
- HL014985/HL/NHLBI NIH HHS/United States
- P01 HL014985/HL/NHLBI NIH HHS/United States
- R01 HL114887/HL/NHLBI NIH HHS/United States
- U01G007674-01/PHS HHS/United States
- P01 HL108800-01/HL/NHLBI NIH HHS/United States
- HL 114887/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical