Role of mitochondrial network stabilisation by a human small heat shock protein in tumour malignancy - PubMed (original) (raw)
. 2015 Mar 25;6(5):470-6.
doi: 10.7150/jca.11494. eCollection 2015.
Affiliations
- PMID: 25874011
- PMCID: PMC4392056
- DOI: 10.7150/jca.11494
Role of mitochondrial network stabilisation by a human small heat shock protein in tumour malignancy
Zsuzsanna Turi et al. J Cancer. 2015.
Abstract
Previously, we found that the unconventional small human heat-shock protein HSPB11 inhibits cell death by HSP90 mediated cholesterol-rich membrane microdomain dependent activation of phosphatidylinositol-3 kinase/protein kinase B pathway and by stabilising the mitochondrial membrane systems. Also, progressive cytoplasmic expression of HSPB11 correlated with brain tumor malignancy. In the present study we investigated how cytoplasmic abundance of HSPB11 augments tumor malignancy. We up- and downregulated the cytoplasmic level of HSPB11 before paclitaxel exposure in NIH3T3 and HeLa cells, which normally express low and high levels, respectively, of the HSPB11 protein. We examined the paclitaxel-mediated induction of cell death, mitochondrial fission, HSPB11 mitochondrial translocation and inhibitory phosphorylation of dynamin-like protein-1 (DLP1). We found that increasing cytoplasmic abundance of HSPB11 in NIH3T3 cells protected against paclitaxel-induced apoptosis, while suppressing HSPB11 in HeLa cells sensitised the cells toward paclitaxel. Also, paclitaxel enhanced mitochondrial translocation of HSPB11 in wild type HeLa but not in NIH3T3 cells. More importantly, increased cytoplasmic level of HSPB11 in NIH3T3 cells enhanced the inhibitory phosphorylation of DLP1 and attenuated paclitaxel-induced mitochondrial fission. All these results suggest that increased cytoplasmic abundance of HSPB11 augments inhibitory phosphorylation of DLP1 thereby reduces mitochondrial fission that eventually leads to decreased apoptosis. This novel mechanism may explain the resistance to apoptosis and increased malignancy of HSPB11-overexpressing tumours. The clinical significance of this mechanism has already been highlighted by the finding that the kinase inhibitor tyrphostin A9 induces cancer cell death by DLP1-mediated mitochondrial fragmentation.
Keywords: DLP1; HSPB11; HeLa; apoptosis; cytoprotection; mitochondrial fission; sHSP; tumour malignancy..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Figure 1
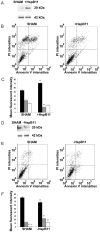
Effect of HspB11 on paclitaxel-induced cell death. NIH3T3 fibroblasts (A, B, C) and HeLa cervical carcinoma cells (D, E, F) were transfected with empty (SHAM), HspB11-expressing (+HspB11) or HspB11-silencing (-HspB11) plasmids. The expression of HspB11 (A, D) was determined using immunoblotting with a custom-made polyclonal anti-HspB11 primary antibody. Actin was used as a loading control. After transfection, the cells were exposed to 150 nM paclitaxel for 3 h and double-stained with fluorescein-conjugated Annexin V and PI, and necrosis and apoptosis were assessed using flow cytometry. Representative dot-plots (B, E) and bar diagrams (C, F) of three independent experiments are presented. The horizontal and vertical axes of the dot-plots represent the Annexin V and PI staining intensities, respectively. Lower left quadrant, living cells; lower right quadrant, early apoptotic cells; upper left quadrant, necrotic cells; upper right quadrant, late apoptotic cells. The bar diagrams represent living (black bars), apoptotic (grey bars) and necrotic (white bars) cells. The values are the mean ± SEM, and significant differences compared with the appropriate SHAM values are indicated as * (p<0.05).
Figure 2
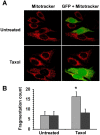
Effects of HspB11 and paclitaxel on mitochondrial fission. NIH3T3 fibroblasts were transiently transfected with a plasmid expressing a HspB11-GFP fusion protein and were exposed to 150 nM paclitaxel (Taxol) or not exposed (Untreated) for 3 h. After loading of the red fluorescent mitochondrial dye MitoTracker Red, mitochondria and GFP were visualised using laser scanning confocal microscopy. Representative images (A) of three independent experiments are presented. The left (red channel) and right (merged red and green channels) panels depict the same microscopic field. The HspB11-overexpressing cells are green-yellow in the right panels, whereas the wild-type cells are red. Mitochondrial fragmentation counts (B) were determined from the MitoTracker Red images, and mean ± SEM fragmentation values of randomly selected 25 cells (per group) are presented as a bar diagram. Significant differences between SHAM (light bars) and transfected (dark bars) are indicated as * (p<0.05).
Figure 3
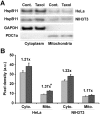
Effects of paclitaxel on HspB11 translocation. After subcellular fractionation, the cytoplasmic (Cyto.) and mitochondrial (Mito.) levels of HspB11 in paclitaxel-treated (dark bars) and untreated (Cont. and light bars) HeLa and NIH3T3 cells were assessed using immunoblotting with a custom-made, polyclonal anti-HspB11 primary antibody. Representative blots (A) and bar diagrams (B) of three independent experiments are presented. In addition to the anti-HspB11 antibody, subcellular fractions were also stained with anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and anti-pyruvate dehydrogenase complex-1α (PDC1a) primary antibodies to indicate the purity of the subcellular fraction and serve as loading controls. The values represent the mean ± SEM, the Taxol per Cont. values are indicated above the bars, and significant differences from the paclitaxel-induced increase in the cytoplasmic level of HspB11 in HeLa cells are indicated as * (p<0.05).
Figure 4
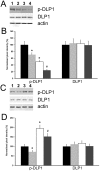
Effect of HspB11 on paclitaxel-induced dephosphorylation of DLP1. HeLa cervical carcinoma cells (A, B) and NIH3T3 fibroblasts (C, D) were transfected with empty, HspB11-silencing (A, B) and HspB11-expressing plasmids (C, D) and were then exposed or not to 150 nM paclitaxel for 3 h. The expression and phosphorylation state of DLP1 were assessed using immunoblotting with anti-DLP1 and phospho(Ser637)-specific anti-DLP1 primary antibodies, respectively, in four experimental groups: sham-transfected-untreated (1 and black bars), sham-transfected-paclitaxel-treated (2 and light grey bars), transfected-untreated (3 and white bars) and transfected-paclitaxel-treated (4 and dark grey bars). Representative blots (A, C) and bar diagrams (B, D) from three independent experiments are presented. Actin was used as a loading control, and the pixel densities were normalised to the appropriate actin value. The values represent the mean ± SEM; significant differences from the sham-transfected-untreated group are indicated as * (p<0.05). Significant differences from the transfected-untreated groups are indicated as # (p<0.05).
Similar articles
- Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1.
Yu T, Fox RJ, Burwell LS, Yoon Y. Yu T, et al. J Cell Sci. 2005 Sep 15;118(Pt 18):4141-51. doi: 10.1242/jcs.02537. Epub 2005 Aug 23. J Cell Sci. 2005. PMID: 16118244 - DLP1-dependent mitochondrial fragmentation and redistribution mediate prion-associated mitochondrial dysfunction and neuronal death.
Li C, Wang D, Wu W, Yang W, Ali Shah SZ, Zhao Y, Duan Y, Wang L, Zhou X, Zhao D, Yang L. Li C, et al. Aging Cell. 2018 Feb;17(1):e12693. doi: 10.1111/acel.12693. Epub 2017 Nov 22. Aging Cell. 2018. PMID: 29166700 Free PMC article. - Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via Dynamin-like protein.
Niu J, Yu M, Wang C, Xu Z. Niu J, et al. J Neurochem. 2012 Aug;122(3):650-8. doi: 10.1111/j.1471-4159.2012.07809.x. Epub 2012 Jun 22. J Neurochem. 2012. PMID: 22639965 - Association between small heat shock protein B11 and the prognostic value of MGMT promoter methylation in patients with high-grade glioma.
Cheng W, Li M, Jiang Y, Zhang C, Cai J, Wang K, Wu A. Cheng W, et al. J Neurosurg. 2016 Jul;125(1):7-16. doi: 10.3171/2015.5.JNS142437. Epub 2015 Nov 6. J Neurosurg. 2016. PMID: 26544773 - Adrenergic Regulation of Drp1-Driven Mitochondrial Fission in Cardiac Physio-Pathology.
Jhun BS, O-Uchi J, Adaniya SM, Cypress MW, Yoon Y. Jhun BS, et al. Antioxidants (Basel). 2018 Dec 18;7(12):195. doi: 10.3390/antiox7120195. Antioxidants (Basel). 2018. PMID: 30567380 Free PMC article. Review.
Cited by
- HSPB11 is a Prognostic Biomarker Associated with Immune Infiltrates in Hepatocellular Carcinoma.
Liu H, Yang M, Dong Z. Liu H, et al. Int J Gen Med. 2022 Apr 13;15:4017-4027. doi: 10.2147/IJGM.S363679. eCollection 2022. Int J Gen Med. 2022. PMID: 35444459 Free PMC article. - Heat Shock Protein 70 Improves In Vitro Embryo Yield and Quality from Heat Stressed Bovine Oocytes.
Stamperna K, Giannoulis T, Dovolou E, Kalemkeridou M, Nanas I, Dadouli K, Moutou K, Mamuris Z, Amiridis GS. Stamperna K, et al. Animals (Basel). 2021 Jun 16;11(6):1794. doi: 10.3390/ani11061794. Animals (Basel). 2021. PMID: 34208520 Free PMC article. - Dynamins and BAR Proteins-Safeguards against Cancer.
Sundborger AC, Hinshaw JE. Sundborger AC, et al. Crit Rev Oncog. 2015;20(5-6):475-84. doi: 10.1615/CritRevOncog.v20.i5-6.160. Crit Rev Oncog. 2015. PMID: 27279242 Free PMC article. Review. - Small heat shock proteins are induced during multiple sclerosis lesion development in white but not grey matter.
Peferoen LA, Gerritsen WH, Breur M, Ummenthum KM, Peferoen-Baert RM, van der Valk P, van Noort JM, Amor S. Peferoen LA, et al. Acta Neuropathol Commun. 2015 Dec 22;3:87. doi: 10.1186/s40478-015-0267-2. Acta Neuropathol Commun. 2015. PMID: 26694816 Free PMC article.
References
- Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther. 2004;101:227–57. - PubMed
- Arrigo AP, Simon S, Gibert B. et al. Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–74. - PubMed
- Arismendi-Morillo G. Electron microscopy morphology of the mitochondrial network in human cancer. Int J Biochem Cell Biol. 2009;41:2062–68. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous