The Interplay between Alpha-Synuclein Clearance and Spreading - PubMed (original) (raw)
Review
The Interplay between Alpha-Synuclein Clearance and Spreading
Tomás Lopes da Fonseca et al. Biomolecules. 2015.
Abstract
Parkinson's Disease (PD) is a complex neurodegenerative disorder classically characterized by movement impairment. Pathologically, the most striking features of PD are the loss of dopaminergic neurons and the presence of intraneuronal protein inclusions primarily composed of alpha-synuclein (α-syn) that are known as Lewy bodies and Lewy neurites in surviving neurons. Though the mechanisms underlying the progression of PD pathology are unclear, accumulating evidence suggests a prion-like spreading of α-syn pathology. The intracellular homeostasis of α-syn requires the proper degradation of the protein by three mechanisms: chaperone-mediated autophagy, macroautophagy and ubiquitin-proteasome. Impairment of these pathways might drive the system towards an alternative clearance mechanism that could involve its release from the cell. This increased release to the extracellular space could be the basis for α-syn propagation to different brain areas and, ultimately, for the spreading of pathology and disease progression. Here, we review the interplay between α-syn degradation pathways and its intercellular spreading. The understanding of this interplay is indispensable for obtaining a better knowledge of the molecular basis of PD and, consequently, for the design of novel avenues for therapeutic intervention.
Figures
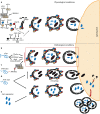
Figure 1
α-syn and the CMA. (A) Under physiological conditions, Hsc70 recognizes the KFERQ domain of α-syn (1) and targets the protein towards the lysosome (2). At the lysosomal membrane, α-syn interacts with LAMP-2A and promotes its oligomerization (3) leading to the entrance of the protein into the lysosome (4). Once inside the lysosome, α-syn is degraded by proteases (5); (B) PTMs such as oxidation and nitration slightly inhibit this pathway and reduce α-syn degradation; (C) Phosphorylation of α-syn on S129 impairs its degradation via CMA. However, while the phosphorylated form of protein does not block this pathway (D), dopamine-modified α-syn and some familial mutations (A30P and A53T) that are also not degraded via CMA, can block this pathway and prevent the degradation of other CMA substrates.
Figure 2
α-syn and Macroautophagy. (A) Macroautophagy is composed of fine-tuned machinery that ensures specific target recognition and cargo delivery to the lysosome; (B) Accumulated α-syn increases mTor and decreases Atg7 levels, promoting mislocalization of Atg9 and leading to impairment of macroautophagy; (C) The α-syn familial mutation E46K can inhibit macroautophagy via JNK/Blc2, an mTor independent pathway; (D) On the other hand, two different effects are associated with the A53T α-syn mutation: an increase in mitophagy, and accumulation of autophagosomes due to impaired degradation; (E) α-syn aggregates cannot be degraded by macroautophagy, leading to the impairment of the pathway.
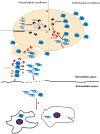
Figure 3
Lysosomal dysfunction can promote the release of miscleaved aggregation-prone α-syn species. (A) Under physiological conditions, CTSD is the main lysosomal enzyme clearing α-syn. (B) Deficient CTSD function promotes α-syn aggregation and lysosomal dysregulation, including the up-regulation of other enzymes such as CTSB that can convert α-syn aggregates into seeding particles able to break and escape from lysosome. (C) Lysosomal rupture releases the content of the organelle (e.g., lysosomal enzymes and toxic α-syn species). (D) These protein particles can be targeted by Calpain1, whose activity, together with that of CTSB, also generates α-syn propagating species. (E) α-syn toxic species can be released to the extracellular space by unknown mechanisms or by cell death derived from lysosomal rupture and be internalized by surrounding cells.
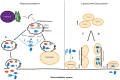
Figure 4
Macrosecretion and lysosome-mediated exocytosis. (A) Since autophagosomes can be secreted to the extracellular space, it is possible that α-syn release occurs via this pathway, both in normal conditions and upon autophagosome accumulation due to lysosome impairment; (B) α-syn can alter the levels of important players in macroautophagy and eventually play a role in macrosecretion; (C) Lysosomes can fuse with the plasma membrane and release their content, a mechanism named lysosome exocytosis. The process is similar to the one observed in neurotransmitter release, α-syn may play a role; (D) Alternatively, impairment of protein degradation might promote the accumulation of high molecular weight species of α-syn inside the lysosome. One can speculate that, as observed in LSDs, the lysosomes might fuse with the plasma membrane and release α-syn oligomers and aggregates into the extracellular media and start the propagation of pathology.
Similar articles
- Autophagy modulates SNCA/α-synuclein release, thereby generating a hostile microenvironment.
Poehler AM, Xiang W, Spitzer P, May VE, Meixner H, Rockenstein E, Chutna O, Outeiro TF, Winkler J, Masliah E, Klucken J. Poehler AM, et al. Autophagy. 2014;10(12):2171-92. doi: 10.4161/auto.36436. Autophagy. 2014. PMID: 25484190 Free PMC article. - Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy.
Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR, Lee VM. Tanik SA, et al. J Biol Chem. 2013 May 24;288(21):15194-210. doi: 10.1074/jbc.M113.457408. Epub 2013 Mar 26. J Biol Chem. 2013. PMID: 23532841 Free PMC article. - Beyond α-synuclein transfer: pathology propagation in Parkinson's disease.
Hansen C, Li JY. Hansen C, et al. Trends Mol Med. 2012 May;18(5):248-55. doi: 10.1016/j.molmed.2012.03.002. Epub 2012 Apr 13. Trends Mol Med. 2012. PMID: 22503115 Review. - Neuronal ApoE Regulates the Cell-to-Cell Transmission of α-Synuclein.
Kang SJ, Kim SJ, Noh HR, Kim BJ, Kim JB, Jin U, Park SA, Park SM. Kang SJ, et al. Int J Mol Sci. 2022 Jul 27;23(15):8311. doi: 10.3390/ijms23158311. Int J Mol Sci. 2022. PMID: 35955451 Free PMC article. - A Cleaning Crew: The Pursuit of Autophagy in Parkinson's Disease.
Parekh P, Sharma N, Gadepalli A, Shahane A, Sharma M, Khairnar A. Parekh P, et al. ACS Chem Neurosci. 2019 Sep 18;10(9):3914-3926. doi: 10.1021/acschemneuro.9b00244. Epub 2019 Aug 20. ACS Chem Neurosci. 2019. PMID: 31385687 Review.
Cited by
- Autophagy mediates proteolysis of NPM1 and HEXIM1 and sensitivity to BET inhibition in AML cells.
Huang M, Garcia JS, Thomas D, Zhu L, Nguyen LX, Chan SM, Majeti R, Medeiros BC, Mitchell BS. Huang M, et al. Oncotarget. 2016 Nov 15;7(46):74917-74930. doi: 10.18632/oncotarget.12493. Oncotarget. 2016. PMID: 27732946 Free PMC article. - Chaperones and Proteostasis: Role in Parkinson's Disease.
Joshi N, Raveendran A, Nagotu S. Joshi N, et al. Diseases. 2020 Jun 22;8(2):24. doi: 10.3390/diseases8020024. Diseases. 2020. PMID: 32580484 Free PMC article. Review. - Extracellular clusterin limits the uptake of α-synuclein fibrils by murine and human astrocytes.
Filippini A, Mutti V, Faustini G, Longhena F, Ramazzina I, Rizzi F, Kaganovich A, Roosen DA, Landeck N, Duffy M, Tessari I, Bono F, Fiorentini C, Greggio E, Bubacco L, Bellucci A, Missale M, Cookson MR, Gennarelli M, Russo I. Filippini A, et al. Glia. 2021 Mar;69(3):681-696. doi: 10.1002/glia.23920. Epub 2020 Oct 12. Glia. 2021. PMID: 33045109 Free PMC article. - Fasudil attenuates aggregation of α-synuclein in models of Parkinson's disease.
Tatenhorst L, Eckermann K, Dambeck V, Fonseca-Ornelas L, Walle H, Lopes da Fonseca T, Koch JC, Becker S, Tönges L, Bähr M, Outeiro TF, Zweckstetter M, Lingor P. Tatenhorst L, et al. Acta Neuropathol Commun. 2016 Apr 22;4:39. doi: 10.1186/s40478-016-0310-y. Acta Neuropathol Commun. 2016. PMID: 27101974 Free PMC article. - Serotonin 1A Receptors on Astrocytes as a Potential Target for the Treatment of Parkinson's Disease.
Miyazaki I, Asanuma M. Miyazaki I, et al. Curr Med Chem. 2016;23(7):686-700. doi: 10.2174/0929867323666160122115057. Curr Med Chem. 2016. PMID: 26795196 Free PMC article. Review.
References
- Baba T., Kikuchi A., Hirayama K., Nishio Y., Hosokai Y., Kanno S., Hasegawa T., Sugeno N., Konno M., Suzuki K., et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: A 3 year longitudinal study. Brain. 2012;135:161–169. doi: 10.1093/brain/awr321. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous