Lassa-vesicular stomatitis chimeric virus safely destroys brain tumors - PubMed (original) (raw)
Lassa-vesicular stomatitis chimeric virus safely destroys brain tumors
Guido Wollmann et al. J Virol. 2015 Jul.
Abstract
High-grade tumors in the brain are among the deadliest of cancers. Here, we took a promising oncolytic virus, vesicular stomatitis virus (VSV), and tested the hypothesis that the neurotoxicity associated with the virus could be eliminated without blocking its oncolytic potential in the brain by replacing the neurotropic VSV glycoprotein with the glycoprotein from one of five different viruses, including Ebola virus, Marburg virus, lymphocytic choriomeningitis virus (LCMV), rabies virus, and Lassa virus. Based on in vitro infections of normal and tumor cells, we selected two viruses to test in vivo. Wild-type VSV was lethal when injected directly into the brain. In contrast, a novel chimeric virus (VSV-LASV-GPC) containing genes from both the Lassa virus glycoprotein precursor (GPC) and VSV showed no adverse actions within or outside the brain and targeted and completely destroyed brain cancer, including high-grade glioblastoma and melanoma, even in metastatic cancer models. When mice had two brain tumors, intratumoral VSV-LASV-GPC injection in one tumor (glioma or melanoma) led to complete tumor destruction; importantly, the virus moved contralaterally within the brain to selectively infect the second noninjected tumor. A chimeric virus combining VSV genes with the gene coding for the Ebola virus glycoprotein was safe in the brain and also selectively targeted brain tumors but was substantially less effective in destroying brain tumors and prolonging survival of tumor-bearing mice. A tropism for multiple cancer types combined with an exquisite tumor specificity opens a new door to widespread application of VSV-LASV-GPC as a safe and efficacious oncolytic chimeric virus within the brain.
Importance: Many viruses have been tested for their ability to target and kill cancer cells. Vesicular stomatitis virus (VSV) has shown substantial promise, but a key problem is that if it enters the brain, it can generate adverse neurologic consequences, including death. We tested a series of chimeric viruses containing genes coding for VSV, together with a gene coding for the glycoprotein from other viruses, including Ebola virus, Lassa virus, LCMV, rabies virus, and Marburg virus, which was substituted for the VSV glycoprotein gene. Ebola and Lassa chimeric viruses were safe in the brain and targeted brain tumors. Lassa-VSV was particularly effective, showed no adverse side effects even when injected directly into the brain, and targeted and destroyed two different types of deadly brain cancer, including glioblastoma and melanoma.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
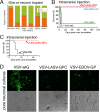
FIG 1
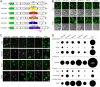
Productive infection of glioma cultures by chimeric VSVs. (A) Schematic shows the gene order, relative size of the genes, and substitution of the VSV glycoprotein (G) gene with glycoprotein genes from 5 other viruses. Glycoproteins from Lassa virus and LCMV are denoted by GPC; those from Ebola and Marburg viruses are denoted GP. (B) In initial experiments, we found that all chimeric viruses infected two human gliomas, U87 and U118, using an MOI of 0.1 and studied at 24 h postinoculation; infection is demonstrated by the GFP reporter. A phase-contrast image is shown below each corresponding image of viral infection indicated by GFP. (C) Representative virus plaques are shown, and relative plaque size is indicated by the GFP fluorescence for U118 glioma, a melanoma (501mel), Vero cells, and control human brain cells. Scale bar in millimeters. Plaques were allowed to develop for 20 to 24 h. In comparison of different viruses on a particular cell type, measurements were all done at the same time. (D) Plaque size was measured as an indicator of virus infection and replication and shown relative to the native VSV-wt. The size of each black circle shows the mean size of 60 randomly chosen and measured plaques 24 h after inoculation. SEM is shown by the black line on the upper right side of each circle.
FIG 2
Chimeric viruses show reduced neuron infection and safety in the brain. (A) Mouse brain cultures were inoculated with VSV-wtG and VSV chimeras (MOI of 5). At 24 hpi, expression of GFP by neurons or glia was analyzed in duplicate cultures, and the relative fraction of neuronal or glial infection is displayed. Noninfected cells were not included. (B) Stereotactic viral injection (3.6 × 104 PFU) into the right striatum of adult Swiss Webster mice with VSV-wtG and VSV-ΔM51 strains (n = 6 each) resulted in strong neurotoxicity and death. In contrast, all mice injected with VSV-LASV-GPC and VSV-EBOV-GP (n = 8 each) survived. (C) Similar intracranial injection of VSV-IFN (n = 7) or VSV-LASV-GPC (n = 4) into striatum. Two additional mice received VSV-IFN in the hypothalamus; both died in 3 days (not included on graph). (D) Human neuronal cultures inoculated with virus (MOI of 1) show significantly reduced infection with VSV-LASV-GPC and VSV-EBOV-GP compared to the strong infection with VSV-wtG.
FIG 3
Survival and oncoselectivity following infection with VSV-LASV-GPC are interferon dependent. (A and B) IFN-α protects nontumor cells from VSV-LASV-GPC infection. Cultures of human U87 glioma (A) and normal cells, including human glia, human fibroblasts, and human neurons (B), were treated with 100 IU/ml IFN-α for 8 h before being infected with VSV-LASV-GPC at an MOI of 0.1. In spite of IFN treatment, strong viral infection was seen on glioma cells at 24 hpi; in contrast, little VSV-LASV-GPC infection was found on nontumor normal human cells. Scale bar, 100 μm. (C) Cultures containing human neurons and glia were infected with VSV-wtG or VSV-LASV-GPC at an MOI of 1 in the presence or absence of 100 IU/ml IFN-α, respectively. IFN had little effect in reducing infection of neurons by VSV-wtG but substantially attenuated infection of neurons by VSV-LASV-GPC (P < 0.05). (D) Intracranial injection of VSV-wtG (n = 3) and VSV-LASV-GPC (n = 5) into IFN-α/β-R−/− mice resulted in death within 3 days (VSV-wtG) and 7 days (VSV-LASV-GPC). VSV-LASV-GPC injection in normal mice (IFN-α/β-R+/+) did not result in neurotoxicity or mortality in any mice. (E to G) Typical microscopic images of injected striatum sections showing VSV-LASV-GPC infection in IFN-α/β-R−/− and IFN-α/β-R+/+ mice. Scale bar, 100 μm. (H) Binding of VSV-wtG and VSV-LASV-GPC was assessed by qRT-PCR following incubation at 4°C, and binding and internalization at 37°C on neurons or U87 glioma cells (n = 3; SEM). (I) Quantification of viral replication in neurons and U87 glioma cells was assessed by plaque assay at 15 and 24 hpi. VSV-wtG produced equal titers of progeny in both neurons and glioma. (J) In contrast, VSV-LASV-GPC progeny production was strongly reduced in neurons compared to human glioma.
FIG 4
Intravenous VSV-LASV-GPC destroys brain glioma and prolongs life indefinitely. CB17 SCID mice with unilateral striatal xenografts of human RFP-expressing rU87 glioma were treated with a single intravenous injection of either VSV-LASV-GPC, VSV-EBOV-GP (each 100 μl of 106 PFU), or saline (control) 15 days after tumor placement. (A) Kaplan-Meier curve showing modest survival benefit of VSV-EBOV-GP-treated mice compared to untreated control (median survival of 29 versus 34 days; n = 8 each group). In contrast, VSV-LASV-GPC-treated mice showed complete survival throughout the observation period (80 days, n = 8). Histological analysis showed no residual viable tumor mass, only scar tissue and tumor cavity (E). (B) The panel displays representative brains for each group. Brains from untreated control mice showed massive expansion of the tumor mass (pink), causing a significant midline shift of the longitudinal cerebral fissure. Large tumor mass and midline shift is also seen in brains from mice treated with VSV-EBOV-GP, indicating limited therapeutic effect. In contrast, brains from VSV-LASV-GPC-treated mice (top row) showed no visible expansion of the brain at the end of the observation period. (C) In control mouse, red glioma expanded substantially. (D) Mouse treated with VSV-EBOV-GP showed selective infection in tumor, but the infection was not complete. (E) Intravenous VSV-LASV-GPC completely eliminated tumor by 79 days. The majority of VSV-LASV-GPC-treated brains showed an empty tumor cavity, indicating successful oncolysis. (F) Syngeneic rat brain tumor model was established by stereotactically grafting RFP-expressing CNS-1 rat glioma into Lewis rats into the right striatum. VSV-LASV-GPC was applied to the area of the tumor 7 days after tumor placement; rats were euthanized 3 days later. VSV-LASV-GPC caused widespread oncolysis in the central portions of CNS-1 tumors with dead or dying cells, resulting in fading of the RFP and GFP signals and a morphological shift to cell debris (open arrowheads in panel G) from the whole-cell architecture seen in noninfected CNS-1 glioma (white arrowheads in panel H). Infection was restricted to the tumor. These data support the view that VSV-LASV-GPC not only targets human glioma but can also selectively infect rodent glioma within the rodent brain.
FIG 5
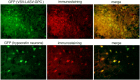
VSV-LASV-G infects widespread gliomas within brain. CB17 SCID mice received bilateral xenografts of RFP-expressing rU87 glioma. Fifteen days later, the right-side tumor was injected stereotactically with 1 μl (3.6 × 104 PFU) of VSV-LASV-GPC. Brains were harvested 8 days later. (A) Analysis for expression of RFP (tumor) and GFP (virus infection) revealed a consistently bright fluorescent signal on the left noninjected side and faint or absent signal together with substantial dead cell glioma debris on the injected right side. These observations suggest successful ipsilateral tumor oncolysis and progressive infection contralateral to the side of virus inoculation. Images show a dorsal view of uncut brains of 4 mice taken with an Olympus fluorescence microscope. (B) Coronal sections from the striatum (from panel A above) showed little residual tumor or infected cells with faint RFP-positive tumor debris at the injected right-side tumor (microscopic images in panel B); the noninjected left side (L) showed VSV-LASV-GPC infection due to contralateral spread of the virus within the brain. The right side (R) showed only tumor remnants remaining after virus infection. Micrographs were made from a dorsal view of the whole brain.
FIG 6
VSV-LASV-GPC targets intracranial melanoma. RFP-expressing rYUMAC melanoma cells were injected bilaterally into the striatum of CB17 SCID mice (see control, left column in panel A) as a model for melanoma metastasis. Fifteen days later, the right-side tumor was injected stereotactically with 1 μl (3.6 × 104 PFU) VSV-LASV-GPC. At 8 dpi, brains were harvested and analyzed for expression of RFP (tumor) and GFP (virus infection). Here, a dorsal view of the whole brain (n = 4) is shown, with red indicating the melanoma and green (GFP) the presence of virus. (B) Coronal sections from the striatum showed little residual tumor or infected cells with faint RFP-positive tumor debris at the injected right-side tumor; the noninjected left side showed VSV-LASV-GPC infection due to contralateral spread of the virus with minimal infection of intervening normal brain. (C and D) VSV-LASV-GPC injected into the right melanoma completely eliminated it and then began to infect and destroy the noninfected left melanoma. (E and F) Higher magnification showing the initial signs of infection in the left melanoma and the complete loss of melanoma cells on the injected side. (G, H, and I) Panel G shows red melanoma cells migrating away from the main tumor body, panel H shows GFP-labeled virus-infected cells, and panel I shows the merged image, demonstrating that only melanoma cells were infected by the virus.
FIG 7
Infection of VSV-LASV-GPC generalizes to a wide variety of human tumors. Eight human tumor lines (see phase-contrast images in left column) originating from bone, breast, prostate, colon, and bladder cancer were infected with VSV-LASV-GPC at an MOI of 3 (primary inoculation). Two hours later, virus inoculum was removed and cells were washed 3 times. At 24 h after infection, all tumor lines showed infection as indicated by GFP expression (middle column). To test for viral propagation in these tumors, supernatant was filtered (0.22 μm) and transferred to uninfected tumor dishes (secondary inoculation; right column). Twenty-four hours later, positive GFP expression indicates transfer of viral progeny produced by tumors infected during primary inoculation.
FIG 8
VSV-LASV-GPC induces humoral response and antibody production against virus and GFP transgene. Serum from mice injected and boosted with VSV-LASV-GPC intranasally (5 × 105 PFU in 50 μl) and intramuscularly (2 × 105 PFU in 20 μl) was diluted and used for immunostaining of cells infected with VSV-LASV-GPC. The top row displays example images of sections of brains from IFN-α/β-R−/− mice showing widespread infection with VSV-LASV-GPC. Antibodies raised in VSV-LASV-GPC-immunized mice labeled infected cell bodies and processes. The same serum was used to test for labeling of a GFP transgene. Sections of transgenic mice expressing GFP in hypocretin neurons were used. In the absence of VSV-LASV-GPC, the antiserum selectively labeled GFP-positive neurons red, indicating that VSV-LASV-GPC evokes an immune response to nonviral antigens associated with the virus. Control nonimmune serum immunostaining was negative.
Similar articles
- Chikungunya-vesicular stomatitis chimeric virus targets and eliminates brain tumors.
Zhang X, Mao G, van den Pol AN. Zhang X, et al. Virology. 2018 Sep;522:244-259. doi: 10.1016/j.virol.2018.06.018. Epub 2018 Jul 26. Virology. 2018. PMID: 30055515 Free PMC article. - Chikungunya, Influenza, Nipah, and Semliki Forest Chimeric Viruses with Vesicular Stomatitis Virus: Actions in the Brain.
van den Pol AN, Mao G, Chattopadhyay A, Rose JK, Davis JN. van den Pol AN, et al. J Virol. 2017 Feb 28;91(6):e02154-16. doi: 10.1128/JVI.02154-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077641 Free PMC article. - Mucin-Like Domain of Ebola Virus Glycoprotein Enhances Selective Oncolytic Actions against Brain Tumors.
Zhang X, Zhang T, Davis JN, Marzi A, Marchese AM, Robek MD, van den Pol AN. Zhang X, et al. J Virol. 2020 Mar 31;94(8):e01967-19. doi: 10.1128/JVI.01967-19. Print 2020 Mar 31. J Virol. 2020. PMID: 32051271 Free PMC article. - [Vesicular stomatitis virus in the fight against cancer].
Janelle V, Poliquin L, Lamarre A. Janelle V, et al. Med Sci (Paris). 2013 Feb;29(2):175-82. doi: 10.1051/medsci/2013292015. Epub 2013 Feb 28. Med Sci (Paris). 2013. PMID: 23452604 Review. French. - A Brighton Collaboration standardized template with key considerations for a benefit/risk assessment for the emergent vesicular stomatitis virus (VSV) viral vector vaccine for Lassa fever.
Spurgers K, Wynn M, Charles L, Hamm S, Matassov D, Gerardi C, Smith ER, Gurwith M, Chen RT; Benefit-Risk Assessment of Vaccines by Technology Working Group (BRAVATO; ex-V3SWG). Spurgers K, et al. Vaccine. 2025 Jun 11;58:127137. doi: 10.1016/j.vaccine.2025.127137. Epub 2025 May 13. Vaccine. 2025. PMID: 40367816 Review.
Cited by
- Reovirus FAST Protein Enhances Vesicular Stomatitis Virus Oncolytic Virotherapy in Primary and Metastatic Tumor Models.
Le Boeuf F, Gebremeskel S, McMullen N, He H, Greenshields AL, Hoskin DW, Bell JC, Johnston B, Pan C, Duncan R. Le Boeuf F, et al. Mol Ther Oncolytics. 2017 Aug 4;6:80-89. doi: 10.1016/j.omto.2017.08.001. eCollection 2017 Sep 15. Mol Ther Oncolytics. 2017. PMID: 28856238 Free PMC article. - Chikungunya-vesicular stomatitis chimeric virus targets and eliminates brain tumors.
Zhang X, Mao G, van den Pol AN. Zhang X, et al. Virology. 2018 Sep;522:244-259. doi: 10.1016/j.virol.2018.06.018. Epub 2018 Jul 26. Virology. 2018. PMID: 30055515 Free PMC article. - Oncolytic Alphaviruses in Cancer Immunotherapy.
Lundstrom K. Lundstrom K. Vaccines (Basel). 2017 Apr 12;5(2):9. doi: 10.3390/vaccines5020009. Vaccines (Basel). 2017. PMID: 28417936 Free PMC article. Review. - Lassa-VSV chimeric virus targets and destroys human and mouse ovarian cancer by direct oncolytic action and by initiating an anti-tumor response.
van den Pol AN, Zhang X, Lima E, Pitruzzello M, Albayrak N, Alvero A, Davis JN, Mor G. van den Pol AN, et al. Virology. 2021 Mar;555:44-55. doi: 10.1016/j.virol.2020.10.009. Epub 2020 Nov 12. Virology. 2021. PMID: 33453650 Free PMC article. - Oncolytic Virus with Attributes of Vesicular Stomatitis Virus and Measles Virus in Hepatobiliary and Pancreatic Cancers.
Nagalo BM, Breton CA, Zhou Y, Arora M, Bogenberger JM, Barro O, Steele MB, Jenks NJ, Baker AT, Duda DG, Roberts LR, Russell SJ, Peng KW, Borad MJ. Nagalo BM, et al. Mol Ther Oncolytics. 2020 Sep 25;18:546-555. doi: 10.1016/j.omto.2020.08.007. Epub 2020 Aug 19. Mol Ther Oncolytics. 2020. PMID: 32839735 Free PMC article.
References
- Croteau D, Mikkelsen T, Rempel SA, Bogler O, Rosenblum M. 2001. New innovations and developments for glioma treatment. Clin Neurosurg 48:60–81. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA161048/CA/NCI NIH HHS/United States
- R01 CA175577/CA/NCI NIH HHS/United States
- R01 CA188359/CA/NCI NIH HHS/United States
- CA188359/CA/NCI NIH HHS/United States
- NS083848/NS/NINDS NIH HHS/United States
- F31 AG041582/AG/NIA NIH HHS/United States
- R01 NS083848/NS/NINDS NIH HHS/United States
- NIH CA161048/CA/NCI NIH HHS/United States
- CA175577/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical