A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice - PubMed (original) (raw)
A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice
Latha Devi et al. Mol Brain. 2015.
Abstract
Background: Accumulating evidence indicates that partial inhibition of β-site APP-cleaving enzyme 1 (BACE1), which initiates amyloid-β (Aβ) production, mitigates Alzheimer's disease (AD)-like pathologies and memory deficits in a battery of transgenic mouse models. However, our previous investigations suggest that therapeutic BACE1 suppression may be beneficial only if targeted on earlier stages of AD and encounter dramatic reductions in efficacy during disease progression. This study was designed to test the possibility that a combination approach, aimed at inhibiting BACE1 and boosting neprilysin (a major Aβ-degrading enzyme) activities, may be able to mechanistically overcome the limited efficacy of anti-Aβ therapy in advanced AD.
Results: After crossbreeding between BACE1 heterozygous knockout (BACE1(+/-)), neprilysin transgenic (NEP) and 5XFAD mice, we analyzed the resultant mice at 12 months of age when 5XFAD controls showed robust amyloid-β (Aβ) accumulation and elevation of BACE1 expression (~2 folds). Although haploinsufficiency lowered BACE1 expression by ~50% in concordance with reduction in gene copy number, profound β-amyloidosis, memory deficits and cholinergic neuron death were no longer rescued in BACE1(+/-) · 5XFAD mice concomitant with their persistently upregulated BACE1 (i.e., equivalent to wild-type control levels). Notably, neprilysin overexpression not only prevented Aβ accumulation but also suppressed the translation initiation factor eIF2α-associated elevation of BACE1 and lowered levels of the β-secretase-cleaved C-terminal fragment of APP (C99) in NEP · 5XFAD mice. Interestingly, these markers for β-amyloidogenesis in BACE1(+/-) · NEP · 5XFAD mice were further reduced to the levels reflecting a combination of single BACE1 allele ablation and the abolishment of translational BACE1 upregulation. However, since neprilysin overexpression was striking (~8-fold relative to wild-type controls), memory impairments, cholinergic neuronal loss and β-amyloidosis were similarly prevented in NEP · 5XFAD and BACE1(+/-) · NEP · 5XFAD mice.
Conclusions: Our findings indicate that robust overexpression of neprilysin is sufficient to ameliorate AD-like phenotypes in aged 5XFAD mice. We also found that Aβ-degrading effects of overexpressed neprilysin can block deleterious BACE1-elevating mechanisms that accelerate Aβ production, warranting further study to test whether interventions moderately activating neprilysin may be useful for boosting the limited efficacy of therapeutic BACE1 inhibition in treating AD with established Aβ pathology.
Figures
Figure 1
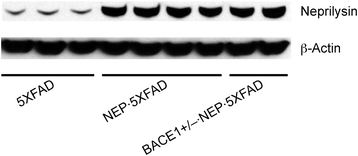
Western blot analysis of transgenic overexpression of neprilysin in 5XFAD mice. Equivalent levels of neprilysin are overexpressed in NEP · 5XFAD and BACE1+/− · NEP · 5XFAD mouse brains (~8-fold relative to 5XFAD controls).
Figure 2
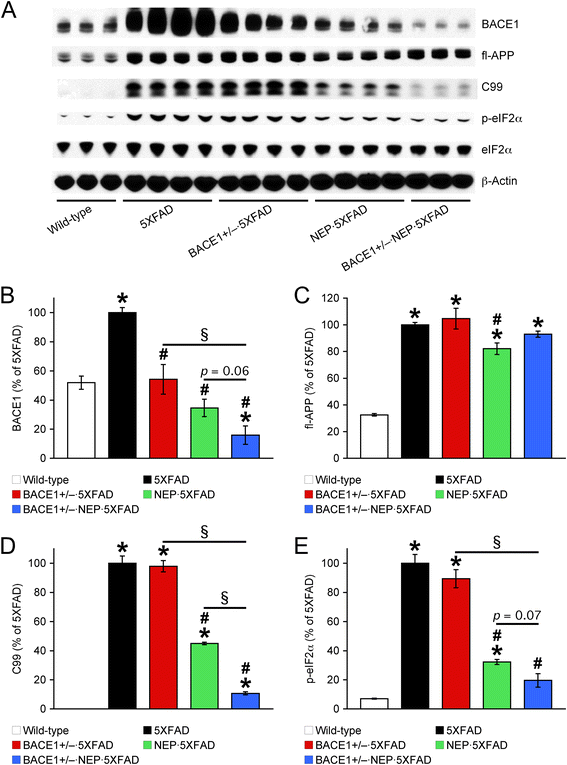
Effects of a combination of BACE1 haploinsufficiency and neprilysin overexpression on β-amyloidogenic processing of APP in 12-month-old 5XFAD mice. (A) Representative immunoblots of protein extracts from hemibrain homogenates of mice. (B–E) Immunoreactive bands were quantified and expressed as the percentage of 5XFAD control mice (n = 3–4 mice per group). Note that BACE1 expression is reduced below wild-type controls in BACE1+/− · NEP · 5XFAD mice, as expected by a single BACE1 allele ablation and the complete abolishment of eIF2α phosphorylation-dependent translational upregulation. Consequently, the β-secretase-cleaved C-terminal fragment of APP (C99) is also dramatically reduced in these mice. * p < 0.05 vs. wild-type, # p < 0.05 vs. 5XFAD, § p < 0.05 vs. BACE1+/− · NEP · 5XFAD. All data are presented as mean ± SEM.
Figure 3
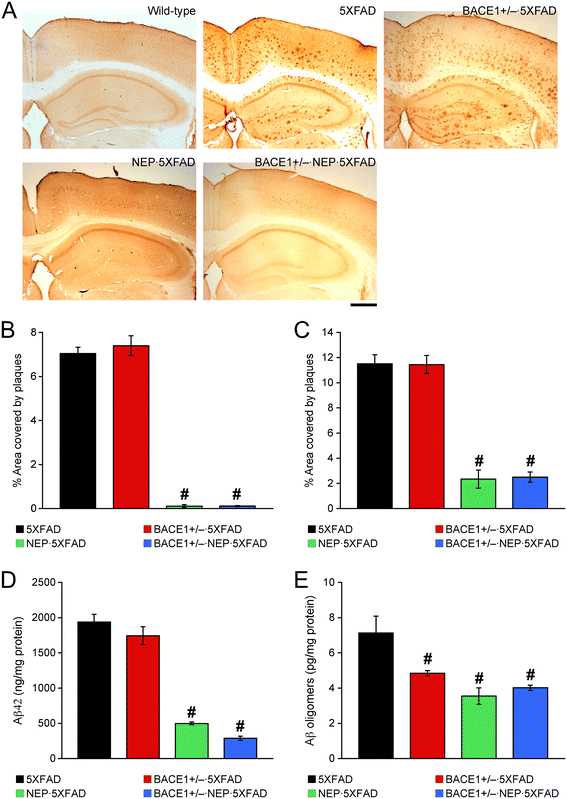
Effects of a combination of BACE1 haploinsufficiency and neprilysin overexpression on β-amyloidosis in 12-month-old 5XFAD mice. (A) Brain sections were immunostained with the 6E10 anti-Aβ antibody. Shown are representative photomicrographs of the hippocampal and cortical regions. Scale bar = 500 μm. (B, C) Percentage area occupied by Aβ deposits in the hippocampus (B) and cerebral cortex (C) was measured for quantification (n = 2–5 mice per group). (D, E) Levels of total Aβ42 in guanidine extracts (D) and soluble Aβ oligomers (E) were quantified by sandwich ELISAs and expressed in nanograms and picograms per milligram of total protein, respectively (n = 4–6 mice per group). Significant and equivalent reductions in all Aβ measurements are observed in NEP · 5XFAD and BACE1+/− · NEP · 5XFAD mice. # p < 0.05 vs. 5XFAD. All data are presented as mean ± SEM.
Figure 4
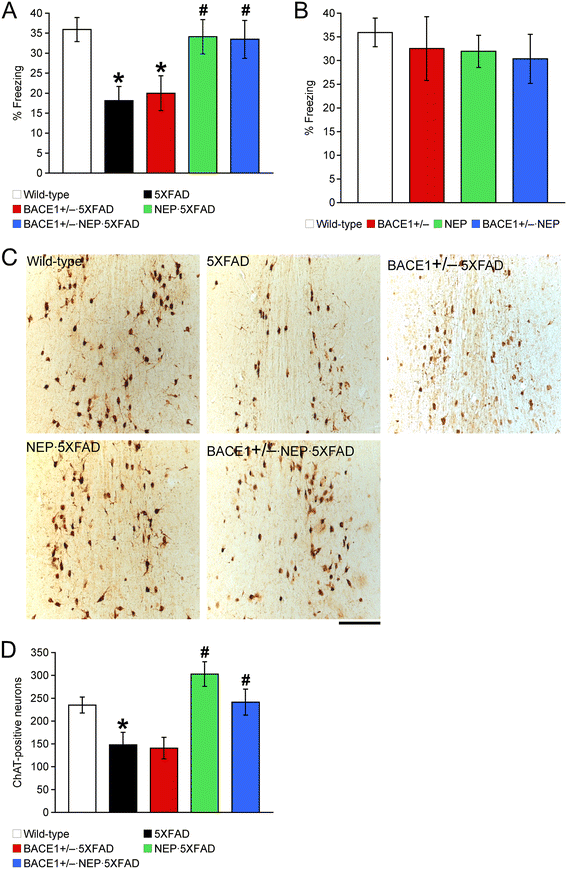
Effects of a combination of BACE1 haploinsufficiency and neprilysin overexpression on memory deficits and cholinergic neuron loss in 12-month-old 5XFAD mice. (A, B) Mice were trained with a CS-US pairing for contextual fear conditioning (n = 6–11 mice per group). 5XFAD mice showed significantly lower levels of contextual freezing than wild-type mice when tested 24 h after training. NEP · 5XFAD and BACE1+/− · NEP · 5XFAD mice are rescued almost completely back to wild-type control levels of contextual memory. (C) Brain sections were immunostained for the cholinergic marker ChAT. Shown are representative photomicrographs of ChAT-immunoreactive neurons in the medial septum of mice. Scale bar = 200 μm. (D) The number of ChAT-positive neurons in the medial septum and the vertical limb of the diagonal band (Ch1/2), which provide the cholinergic innervation to the hippocampus, was counted for quantification (n = 2–5 mice per group). Cholinergic neuronal death associated with 5XFAD is prevented in NEP · 5XFAD and BACE1+/− · NEP · 5XFAD mice. * p < 0.05 vs. wild-type, # p < 0.05 vs. 5XFAD. All data are presented as mean ± SEM.
Similar articles
- Mechanisms that lessen benefits of β-secretase reduction in a mouse model of Alzheimer's disease.
Devi L, Ohno M. Devi L, et al. Transl Psychiatry. 2013 Jul 23;3(7):e284. doi: 10.1038/tp.2013.59. Transl Psychiatry. 2013. PMID: 23880880 Free PMC article. - Effects of BACE1 haploinsufficiency on APP processing and Aβ concentrations in male and female 5XFAD Alzheimer mice at different disease stages.
Devi L, Ohno M. Devi L, et al. Neuroscience. 2015 Oct 29;307:128-37. doi: 10.1016/j.neuroscience.2015.08.037. Epub 2015 Aug 24. Neuroscience. 2015. PMID: 26314636 Free PMC article. - [Elucidating Pathogenic Mechanisms of Early-onset Alzheimer's Disease in Down Syndrome Patients].
Asai M, Kawakubo T, Mori R, Iwata N. Asai M, et al. Yakugaku Zasshi. 2017;137(7):801-805. doi: 10.1248/yakushi.16-00236-2. Yakugaku Zasshi. 2017. PMID: 28674290 Review. Japanese. - Understanding molecular mechanisms of proteolysis in Alzheimer's disease: progress toward therapeutic interventions.
Higuchi M, Iwata N, Saido TC. Higuchi M, et al. Biochim Biophys Acta. 2005 Aug 1;1751(1):60-7. doi: 10.1016/j.bbapap.2005.02.013. Epub 2005 Mar 17. Biochim Biophys Acta. 2005. PMID: 16054018 Review.
Cited by
- EFAD transgenic mice as a human APOE relevant preclinical model of Alzheimer's disease.
Tai LM, Balu D, Avila-Munoz E, Abdullah L, Thomas R, Collins N, Valencia-Olvera AC, LaDu MJ. Tai LM, et al. J Lipid Res. 2017 Sep;58(9):1733-1755. doi: 10.1194/jlr.R076315. Epub 2017 Apr 7. J Lipid Res. 2017. PMID: 28389477 Free PMC article. Review. - Effects of neural stem cell transplantation in Alzheimer's disease models.
Hayashi Y, Lin HT, Lee CC, Tsai KJ. Hayashi Y, et al. J Biomed Sci. 2020 Jan 27;27(1):29. doi: 10.1186/s12929-020-0622-x. J Biomed Sci. 2020. PMID: 31987051 Free PMC article. Review. - Dual therapy for Aβ amyloidosis in AD: A successful one-two combo.
Patel TK, Holtzman DM. Patel TK, et al. J Exp Med. 2018 May 7;215(5):1267-1268. doi: 10.1084/jem.20180494. Epub 2018 Apr 23. J Exp Med. 2018. PMID: 29685951 Free PMC article. - Therapeutic approaches in Parkinson's disease and related disorders.
Valera E, Masliah E. Valera E, et al. J Neurochem. 2016 Oct;139 Suppl 1(Suppl 1):346-352. doi: 10.1111/jnc.13529. Epub 2016 Feb 10. J Neurochem. 2016. PMID: 26749150 Free PMC article. Review. - The Lesion Analysis of Cholinergic Neurons in 5XFAD Mouse Model in the Three-Dimensional Level of Whole Brain.
Yan H, Pang P, Chen W, Zhu H, Henok K A, Li H, Wu Z, Ke X, Wu J, Zhang T, Pan K, Pei L, Han Y, Lu Y. Yan H, et al. Mol Neurobiol. 2018 May;55(5):4115-4125. doi: 10.1007/s12035-017-0621-4. Epub 2017 Jun 8. Mol Neurobiol. 2018. PMID: 28597200
References
- Ohno M. β-Secretase as a prime therapeutic target for Alzheimer’s disease: a perspective from mouse model studies. In: Araki W, editor. Recent Advances in the Biology of Secretases, Key Proteases in Alzheimer’s Disease. Kerala: Research Signpost; 2008. pp. 1–25.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical