Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model - PubMed (original) (raw)
doi: 10.1186/s12866-015-0400-1.
Rebeca Martín 1 2 3, Florian Chain 6 7, Jane M Natividad 8, Jennifer Jury 9, Jun Lu 10, Harry Sokol 11 12 13 14, Vassilia Theodorou 15, Premysl Bercik 16, Elena F Verdu 17, Philippe Langella 18 19 20, Luis G Bermúdez-Humarán 21 22
Affiliations
- PMID: 25888448
- PMCID: PMC4391109
- DOI: 10.1186/s12866-015-0400-1
Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model
Rebeca Martín et al. BMC Microbiol. 2015.
Abstract
Background: The human gut houses one of the most complex and abundant ecosystems composed of up to 10(13)-10(14) microorganisms. The importance of this intestinal microbiota is highlighted when a disruption of the intestinal ecosystem equilibrium appears (a phenomenon called dysbiosis) leading to an illness status, such as inflammatory bowel diseases (IBD). Indeed, the reduction of the commensal bacterium Faecalibacterium prausnitzii (one of the most prevalent intestinal bacterial species in healthy adults) has been correlated with several diseases, including IBD, and most importantly, it has been shown that this bacterium has anti-inflammatory and protective effects in pre-clinical models of colitis. Some dysbiosis disorders are characterized by functional and physiological alterations. Here, we report the beneficial effects of F. prausnitzii in the physiological changes induced by a chronic low-grade inflammation in a murine model. Chronic low-grade inflammation and gut dysfunction were induced in mice by two episodes of dinitro-benzene sulfonic acid (DNBS) instillations. Markers of inflammation, gut permeability, colonic serotonin and cytokine levels were studied. The effects of F. prausnitzii strain A2-165 and its culture supernatant (SN) were then investigated.
Results: No significant differences were observed in classical inflammation markers confirming that inflammation was subclinical. However, gut permeability, colonic serotonin levels and the colonic levels of the cytokines IL-6, INF-γ, IL-4 and IL-22 were higher in DNBS-treated than in untreated mice. Importantly, mice treated with either F. prausnitzii or its SN exhibited significant decreases in intestinal permeability, tissue cytokines and serotonin levels.
Conclusions: Our results show that F. prausnitzii and its SN had beneficial effects on intestinal epithelial barrier impairment in a chronic low-grade inflammation model. These observations confirm the potential of this bacterium as a novel probiotic treatment in the management of gut dysfunction and low-grade inflammation.
Figures
Figure 1
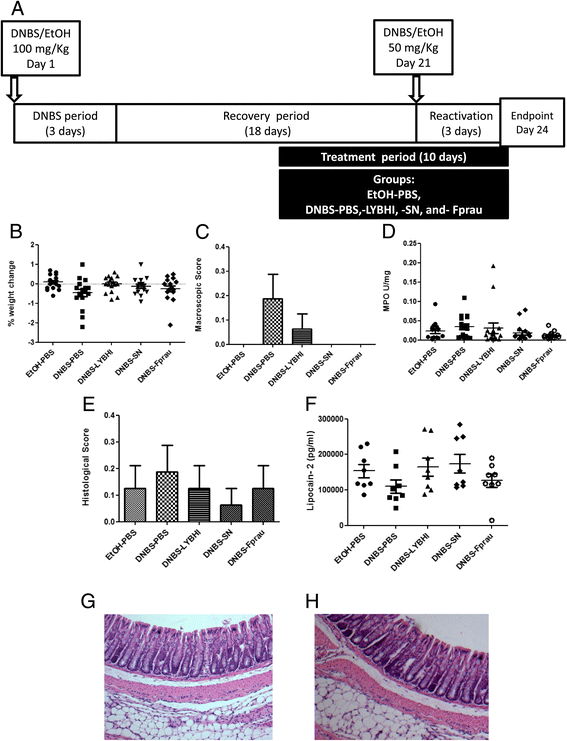
Experimental protocols for the mouse model of chronic micro-inflammation and absence of inflammation. A) Colitis was induced by intrarectal administration of 100 mg/Kg of DNBS in solution in 30% ethanol (EtOH). Control mice (without colitis) received only 30% EtOH. The effects of DNBS are highest during the first 3 days after its administration (DNBS period). Ten days after the end of the DNBS period, bacterial culture, PBS, or supernatant (SN) were intragastrically administered daily for 10 days (gavage period). Colitis was reactivated 21 days after the first DNBS injection with a second injection of 50 mg/Kg of DNBS solution. Three days after reactivation mice were sacrificed. The severity of the reactivated colitis was assessed from difference in body weight (B), macroscopic score (C), MPO activity (D), histological score (E) (n = 16 mice per group) and lipocalin-2 levels (F) (n = 2*4 = 8 mice per group) between control non-inflamed (EtOH-PBS), control inflamed (DNBS-PBS), bacteria-free culture medium (DNBS-LYBHI), F. prausnitzii strain A2-165 (DNBS-Fprau), and F. prausnitzii A2-165 SN (DNBS-SN) groups. Experiments were performed at least in duplicate. Normal appearance of the colon of a control mouse with no inflammation (G) and of a mouse with micro-inflammation (H).
Figure 2
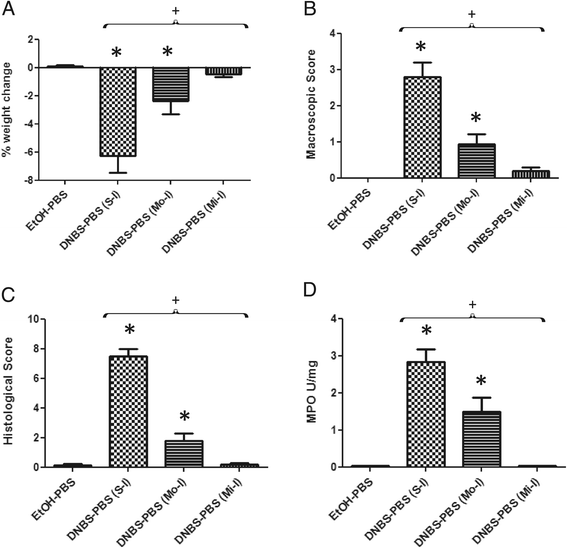
Comparison of inflammation measures in severe and moderate colitis models [25] with the micro-inflammation model employed in this study. Severity of the colitis was assessed from differences in body weight (A), macroscopic score (B), histological score (C) and MPO activity (D) between the control non-inflamed (EtOH-PBS), control inflamed severe colitis model (DNBS-PBS (S-I)) [25], moderate colitis model (DNBS-PBS (M-I)) [25] and micro-inflammation model (DNBS-PBS (Mi-I)). Experiments were performed at least in duplicate. Comparisons involved the non-parametric Kruskal-Wallis test was used followed by a Dunn’s Multiple Comparison test. *p < 0.05 vs. DNBS-PBS, +p < 0.05 (n = 16 mice per group).
Figure 3
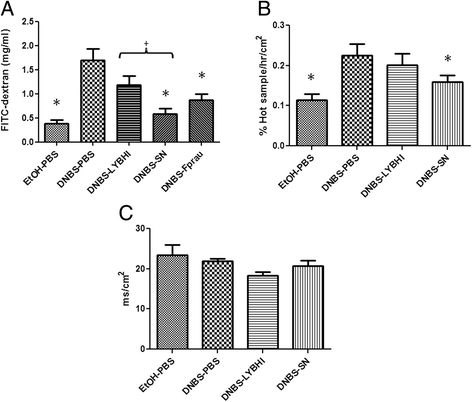
In vivo and in vitro permeability measurements. For in vivo measurements of gut permeability, animals were orally gavaged with FITC-dextran (A) (n = 16 mice per group). For in vitro measures, sections of colon were mounted in Ussing chambers and 51Cr-EDTA flux (% Hot Sample/hr/cm2) (B) and tissue conductance (mS/cm2) (C) were measured (n = 6 mice per group). Control non-inflamed (EtOH-PBS), control inflamed (DNBS-PBS), bacteria-free culture medium (DNBS-LYBHI), F. prausnitzii strain A2-165 (DNBS-Fprau), and F. prausnitzii A2-165 SN (DNBS-SN) groups. Experiments were performed at least in duplicate. Comparisons involved the non-parametric Kruskal-Wallis test was used followed by a Dunn’s Multiple Comparison test. *p < 0.05 vs. DNBS-PBS, +p < 0.05.
Figure 4
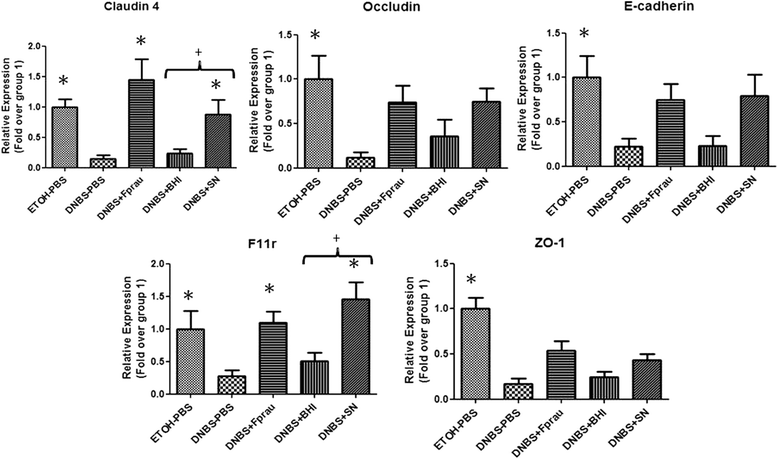
Effect of F. prausnitzii on apical junction proteins mRNAs in a DNBS-induced low-grade inflammation model. Apical junction protein expression levels were determined by real-time qPCR. Control non-inflamed (EtOH-PBS), control inflamed (DNBS-PBS), bacteria-free culture medium (DNBS-LYBHI), F. prausnitzii strain A2-165 (DNBS-Fprau), and F. prausnitzii A2-165 SN (DNBS-SN) groups. Experiments were performed at least in duplicate. Comparisons involved the non-parametric Kruskal-Wallis test was used followed by a Dunn’s Multiple Comparison test. *p < 0.05 vs. DNBS-PBS, +p < 0.05 (n = 6 mice per group).
Figure 5
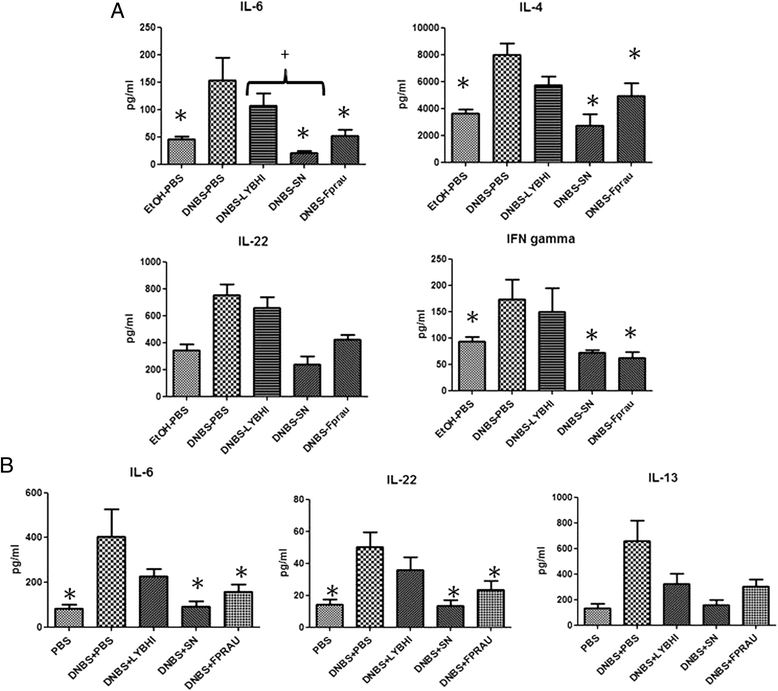
Cytokine concentrations in colon (A) and serum (B) in the DNBS micro-inflammation model. Control non-inflamed (EtOH-PBS), control inflamed (DNBS-PBS), bacteria-free culture medium (DNBS-LYBHI), F. prausnitzii strain A2-165 (DNBS-Fprau), and F. prausnitzii A2-165 SN (DNBS-SN) groups. Experiments were performed at least in duplicate. Comparisons involved the non-parametric Kruskal-Wallis test was used followed by a Dunn’s Multiple Comparison test. *p < 0.05 vs. DNBS-PBS, +p < 0.05 (n = 16 mice per group).
Figure 6
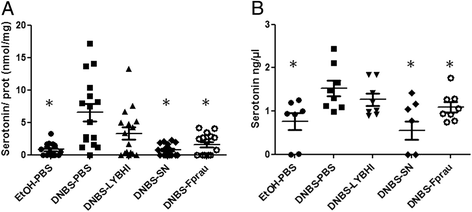
Serotonin concentrations in colon (A) and colon contents (B) in the DNBS micro-inflammation model. Control non-inflamed (EtOH-PBS), control inflamed (DNBS-PBS), bacteria-free culture medium (DNBS-LYBHI), F. prausnitzii strain A2-165 (DNBS-Fprau), and F. prausnitzii A2-165 SN (DNBS-SN) groups. Experiments were performed at least in duplicate.Comparisons involved the non-parametric Kruskal-Wallis test was used followed by a Dunn’s Multiple Comparison test. *p < 0.05 vs. DNBS-PBS, +p < 0.05 (n = 16 mice per group for analysis of colon samples and n = 8 mice per group for analysis of colon content samples).
Similar articles
- Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease.
Quévrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, Miquel S, Carlier L, Bermúdez-Humarán LG, Pigneur B, Lequin O, Kharrat P, Thomas G, Rainteau D, Aubry C, Breyner N, Afonso C, Lavielle S, Grill JP, Chassaing G, Chatel JM, Trugnan G, Xavier R, Langella P, Sokol H, Seksik P. Quévrain E, et al. Gut. 2016 Mar;65(3):415-425. doi: 10.1136/gutjnl-2014-307649. Epub 2015 Jun 4. Gut. 2016. PMID: 26045134 Free PMC article. - The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models.
Martín R, Chain F, Miquel S, Lu J, Gratadoux JJ, Sokol H, Verdu EF, Bercik P, Bermúdez-Humarán LG, Langella P. Martín R, et al. Inflamm Bowel Dis. 2014 Mar;20(3):417-30. doi: 10.1097/01.MIB.0000440815.76627.64. Inflamm Bowel Dis. 2014. PMID: 24418903 - Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis.
Qiu X, Zhang M, Yang X, Hong N, Yu C. Qiu X, et al. J Crohns Colitis. 2013 Dec;7(11):e558-68. doi: 10.1016/j.crohns.2013.04.002. Epub 2013 May 2. J Crohns Colitis. 2013. PMID: 23643066 - Faecalibacterium prausnitzii and human intestinal health.
Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Miquel S, et al. Curr Opin Microbiol. 2013 Jun;16(3):255-61. doi: 10.1016/j.mib.2013.06.003. Epub 2013 Jul 3. Curr Opin Microbiol. 2013. PMID: 23831042 Review. - The critical role of Faecalibacterium prausnitzii in human health: An overview.
Leylabadlo HE, Ghotaslou R, Feizabadi MM, Farajnia S, Moaddab SY, Ganbarov K, Khodadadi E, Tanomand A, Sheykhsaran E, Yousefi B, Kafil HS. Leylabadlo HE, et al. Microb Pathog. 2020 Dec;149:104344. doi: 10.1016/j.micpath.2020.104344. Epub 2020 Jun 11. Microb Pathog. 2020. PMID: 32534182 Review.
Cited by
- Sociability in a non-captive macaque population is associated with beneficial gut bacteria.
Johnson KV, Watson KK, Dunbar RIM, Burnet PWJ. Johnson KV, et al. Front Microbiol. 2022 Nov 11;13:1032495. doi: 10.3389/fmicb.2022.1032495. eCollection 2022. Front Microbiol. 2022. PMID: 36439813 Free PMC article. - Next-Generation Probiotics: Microflora Intervention to Human Diseases.
Zhang H, Duan Y, Cai F, Cao D, Wang L, Qiao Z, Hong Q, Li N, Zheng Y, Su M, Liu Z, Zhu B. Zhang H, et al. Biomed Res Int. 2022 Nov 16;2022:5633403. doi: 10.1155/2022/5633403. eCollection 2022. Biomed Res Int. 2022. PMID: 36440358 Free PMC article. Review. - Isomaltulose Exhibits Prebiotic Activity, and Modulates Gut Microbiota, the Production of Short Chain Fatty Acids, and Secondary Bile Acids in Rats.
Yang ZD, Guo YS, Huang JS, Gao YF, Peng F, Xu RY, Su HH, Zhang PJ. Yang ZD, et al. Molecules. 2021 Apr 23;26(9):2464. doi: 10.3390/molecules26092464. Molecules. 2021. PMID: 33922589 Free PMC article. - Direct impact of commonly used dietary emulsifiers on human gut microbiota.
Naimi S, Viennois E, Gewirtz AT, Chassaing B. Naimi S, et al. Microbiome. 2021 Mar 22;9(1):66. doi: 10.1186/s40168-020-00996-6. Microbiome. 2021. PMID: 33752754 Free PMC article. - Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients.
Ni Y, Lohinai Z, Heshiki Y, Dome B, Moldvay J, Dulka E, Galffy G, Berta J, Weiss GJ, Sommer MOA, Panagiotou G. Ni Y, et al. ISME J. 2021 Nov;15(11):3207-3220. doi: 10.1038/s41396-021-00998-8. Epub 2021 May 17. ISME J. 2021. PMID: 34002024 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources