A Developmental Framework for Graft Formation and Vascular Reconnection in Arabidopsis thaliana - PubMed (original) (raw)
Comment
A Developmental Framework for Graft Formation and Vascular Reconnection in Arabidopsis thaliana
Charles W Melnyk et al. Curr Biol. 2015.
Abstract
Plant grafting is a biologically important phenomenon involving the physical joining of two plants to generate a chimeric organism. It is widely practiced in horticulture and used in science to study the long-distance movement of molecules. Despite its widespread use, the mechanism of graft formation and vascular reconnection is not well understood. Here, we study the dynamics and mechanisms of vascular regeneration in Arabidopsis thaliana during graft formation when the vascular strands are severed and reconnected. We demonstrate a temporal separation between tissue attachment, phloem connection, root growth, and xylem connection. By analyzing cell division patterns and hormone responses at the graft junction, we found that tissues initially show an asymmetry in cell division, cell differentiation, and gene expression and, through contact with the opposing tissue, lose this asymmetry and reform the vascular connection. In addition, we identified genes involved in vascular reconnection at the graft junction and demonstrate that these auxin response genes are required below the graft junction. We propose an inter-tissue communication process that occurs at the graft junction and promotes vascular connection by tissue-specific auxin responses involving ABERRANT LATERAL ROOT FORMATION 4 (ALF4). Our study has implications for phenomena where forming vascular connections are important including graft formation, parasitic plant infection, and wound healing.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
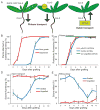
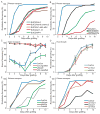
Figure 1. Phloem connection, root growth and xylem connection are temporally separated
A) Cartoons showing the transport assays used. pSUC2::GFP Col-0 scions were grafted to Col-0 rootstocks, and fluorescence monitored in the roots. Alternatively, CFDA was applied to scion or rootstock, and fluorescence monitored in the vascular tissue of the rootstock or scion. B) Plant attachment precedes root growth in grafted Col/Col plants (scion/rootstock notation) 0 to 10 DAG. Mean shown from 5 experiments with 20–36 plants per time point per experiment (+/− SEM). C) Phloem reconnection occurs 3–4 DAG, as monitored by fluorescence appearance in the rootstock after CFDA application to the cotyledons of Col/Col plants. Alternatively, fluorescence was monitored in Col rootstocks grafted to pSUC2::GFP scions. Mean shown from 3–5 experiments with 11–24 plants per time point per experiment (+/− SEM). D) Xylem reconnection occurs 6–7 DAG, as monitored by fluorescence appearance in the scions after CFDA application to the rootstocks of Col/Col or ungrafted plants. Mean shown from 3 experiments with 11–37 plants per time point per experiment (+/− SEM). E) Hydraulic connectivity is restored 7–8 DAG, as monitored by placing Col/Col or ungrafted plants in a low humidity environment and monitoring scion wilting after 24 hours. Mean shown from 3 experiments with 11–37 plants per time point per experiment (+/− SEM).
Figure 2. Cells differentiate and expand upon graft formation
A–C) New xylem vessels (spiral structures denoted by white triangles) form above and below the graft junction (A,B) and in cut shoots (C) as observed with DIC optics of cleared whole mount hypocotyls. For (B), n=10–27 plants per time point. AR – adventitious root. D) In interstock grafts, the phloem reconnects in the top segment first, evident by the presence of GFP signal from the pSUC2::GFP scion in the middle section at 3 DAG. At 4 DAG, the signal continues to the rootstock. Insert shows the fluorescence at the root tip. E) Vascular tissue expands across the graft junction, as observed in a longitudinal-section though a pUBQ10::PM-tdTomato scion grafted to a p35S::YFP-ER rootstock. Inserts above and to the left show orthogonal views of the centre panel (dotted lines). Arrow denotes expanded tissue. Bottom left number represents the number of individuals that showed vascular invasion. F) Epidermal cells expand at both the scion (p35S::GFP-LTi, p35S:: H2B-RFP) and rootstock (p35S::mCherry-LTi, p35S::H2B-YFP) halves of the graft junction. White triangles denote cell expansion 0 to 3 DAG. A–F) Scale bar is 50μm.
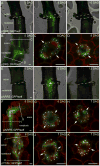
Figure 3. Cell proliferation and gene expression dynamics at the graft junction
A) Transverse-sections 50μm above or 50μm below the graft junction reveal that endodermal cells (white triangles) divide above but not below the graft junction at 3 DAG in pUBQ10::PM-tdTomato expressing plants. B) Autofluorescence of lignin at the graft junction suggests that the lignin-containing Casparian strip network is reformed across the graft junction (dashed line) in many plants at 7 DAG (left panel), but not all (right panel). Bottom left number represents the number of grafts observed with this phenotype. C) A marker of Casparian strip formation, pCASP1::NLS-GFP, is upregulated around the cut site (dashed lines) in grafted plants, but not in ungrafted plants. D) pCASP1::NLS-GFP plants were grafted to themselves or to wild-type plants, and fluorescence intensity of the GFP signal was quantified daily for the same plants. The number of plants above the threshold was plotted to compare pCASP1 activation in the hypocotyl scion versus activation in the rootstock. n = 14–21 per treatment, and representative individuals are shown in Figure 3C and Figure S3. E) Mitotic index in the vascular tissue of plants 2 DAG (n = 10) and 3 DAG (n = 15). Whereas a significant difference in the mitotic index was observed 2 DAG between the scion and rootstock (p<0.001; Welch Two Sample t-test), no difference was found 3 DAG. F) In situ hybridisation showing Histone H4 expression in two grafted plants at 2 DAG and two grafted plants at 3 DAG. Cell divisions (arrowheads) occurred in the vascular tissue close the graft junction (dashed line). G) The wound-responsive marker pWIND1::GFP is upregulated above and below the graft junction upon grafting, and diminishes by 10 DAG. Scale bars 50μm (A–G).
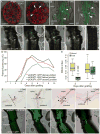
Figure 4. Auxin and cytokinin response are spatially and temporally controlled during vasculature connection
A–D) The auxin-responsive pDR5::GFP-ER gene increases expression at the graft junction upon graft formation and diminishes by 10 DAG (A,B). In transverse-sections 50μm above the graft junction (C), pDR5::GFP-ER expresses highly in the pericycle, whereas 50μm below the graft junction (D), pDR5::GFP-ER expresses highly in the xylem pole pericycle cells. Hypocotyls express pUBQ10::PM-tdTomato (C–D) to outline cell membranes. E–H) The cytokinin-response pARR5::GFP gene increases expression at the graft junction upon graft formation and diminishes by 13 DAG (E,F). In transverse-sections 50μm above and below the graft junction (G,H), pARR5::GFP expresses highly in the pericycle and cambial cells. Hypocotyls express pUBQ10::PM-tdTomato (G,H) or are counterstained with FM4-64 (F). I–K) The cytokinin responsive pTCSn::GFP-ER gene increase expression upon graft formation both above and below the graft junction (I). In transverse-sections 50μm above and below the graft junction (J,K), expression is high in the pericycle and vascular cambium. Hypocotyls express pUBQ10::PM-tdTomato. A–K) Dashed lines represent where the hypocotyl was cut, and solid lines where transverse-sections were made. Asterisks indicate pericycle cells, and arrows point to the orientation of the xylem. Scale bar is 25μm.
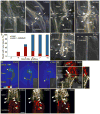
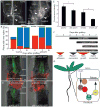
Figure 5. Auxin response genes, including ALF4, are involved specifically in the hypocotyl rootstock for vascular connection
A) Mutations in auxin response genes delay the movement of GFP from wild-type pSUC2::GFP scions into grafted rootstocks. Genotype of the rootstock indicated on the panel. n=24 plants per graft combination. B) Interstock grafts with pSUC2::GFP scions delay GFP movement to the rootstock by one day compared to two segment grafts. A 1mm segment of alf4-1 or axr1-12 mutant tissue in the hypocotyl is sufficient to further delay phloem connection between Col scions and Col rootstocks. Scion/hypocotyl/rootstock genotype indicated. n=24 plants per graft combination. C) alf4-1 affects the transport of CFDA from the rootstock to the scion. Mean shown from 3 experiments with 15–25 plants per time point per experiment (+/− SEM). D) Root growth in Col rootstocks is not affected by the presence of an axr1-12 or alf4-1 scion. Mean shown from 2–8 experiments with 20–25 plants per graft combination per experiment (+/− SEM). E–F) ALF4 and AXR1 are required in the rootstock for efficient movement of CFDA across the graft junction, whereas ALF4 and AXR1 are not required in the scion. A mutant axr1-12 scion can partially rescue the requirement for AXR1 in the root (F). Mean shown from 24 plants per graft combination per time point (E) or from 2 experiments with 20–25 plants per graft combination per time point (F).
Figure 6. ALF4 is involved in auxin-dependent vasculature connection, and a model and time course of graft formation in the Arabidopsis hypocotyl
A) New xylem vessels (spiral structures denoted by white triangles) form above and below the graft junction in wild-type grafts, but formation is enhanced above the graft junction and delayed below the graft junction in alf4/alf4 grafts. Cleared whole mount hypocotyls shown. B) New xylem vessel formation measured in a population of cleared whole mount hypocotyls at various time points after grafting. n=13–27 plants per time point. C–D) ALF4 is required in the rootstock to activate vascular auxin response. Col-0 (C) or alf4 scions (D) in the pDR5::GFP-ER background were grafted to pUBQ10::PM-tdTomato rootstocks, or grafted reciprocally. Scale bar is 25μm. E) Vascular and epidermal auxin response is decreased 20% in alf4-1 compared to Col when scions are grafted to wild-type (WT) rootstocks, whereas it is decreased 49% in alf4-1 compared to Col when rootstocks are grafted to WT scions (*p<0.01; student’s two-sample equal variance t-Test, with a two-tailed distribution). n = 18–20 plants per treatment (+/− SEM). F) A time course of hormone response, cell differentiation and physiological processes associated with hypocotyl graft formation in Arabidopsis seedlings. G) A hypothetical model of graft formation: Auxin produced in the scion moves across the graft junction and triggers an auxin response that involves the auxin receptors (TIR/AFBs) and AXR1. These activate an AUX/IAA-ARF response that may involve IAA18 targets to reconnect the phloem. The precise function of ALF4 is unknown, but this protein may assist in the transport and perception of auxin. Notably, AXR1 and ALF4 are not required in the scion, indicating the scion has a different mechanism for phloem reconnection.
Comment in
- Plant grafting: making the right connections.
Kümpers BM, Bishopp A. Kümpers BM, et al. Curr Biol. 2015 May 18;25(10):R411-3. doi: 10.1016/j.cub.2015.03.055. Curr Biol. 2015. PMID: 25989079
Comment on
- Neuroanatomy: connectome connects fly and mammalian brain networks.
Kaiser M. Kaiser M. Curr Biol. 2015 May 18;25(10):R416-8. doi: 10.1016/j.cub.2015.03.039. Curr Biol. 2015. PMID: 25989081
Similar articles
- Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration.
Melnyk CW, Gabel A, Hardcastle TJ, Robinson S, Miyashima S, Grosse I, Meyerowitz EM. Melnyk CW, et al. Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):E2447-E2456. doi: 10.1073/pnas.1718263115. Epub 2018 Feb 13. Proc Natl Acad Sci U S A. 2018. PMID: 29440499 Free PMC article. - Monitoring Vascular Regeneration and Xylem Connectivity in Arabidopsis thaliana.
Melnyk CW. Melnyk CW. Methods Mol Biol. 2017;1544:91-102. doi: 10.1007/978-1-4939-6722-3_9. Methods Mol Biol. 2017. PMID: 28050832 - Stage-specific events in tomato graft formation and the regulatory effects of auxin and cytokinin.
Cui Q, Xie L, Dong C, Gao L, Shang Q. Cui Q, et al. Plant Sci. 2021 Mar;304:110803. doi: 10.1016/j.plantsci.2020.110803. Epub 2020 Dec 24. Plant Sci. 2021. PMID: 33568302 - The role of plant hormones during grafting.
Nanda AK, Melnyk CW. Nanda AK, et al. J Plant Res. 2018 Jan;131(1):49-58. doi: 10.1007/s10265-017-0994-5. Epub 2017 Nov 27. J Plant Res. 2018. PMID: 29181647 Free PMC article. Review. - Plant grafting: new mechanisms, evolutionary implications.
Goldschmidt EE. Goldschmidt EE. Front Plant Sci. 2014 Dec 17;5:727. doi: 10.3389/fpls.2014.00727. eCollection 2014. Front Plant Sci. 2014. PMID: 25566298 Free PMC article. Review.
Cited by
- Advances in understanding the graft healing mechanism: a review of factors and regulatory pathways.
Wang L, Liao Y, Liu J, Zhao T, Jia L, Chen Z. Wang L, et al. Hortic Res. 2024 Jun 20;11(8):uhae175. doi: 10.1093/hr/uhae175. eCollection 2024 Aug. Hortic Res. 2024. PMID: 39108577 Free PMC article. - Wound signaling: The missing link in plant regeneration.
Chen L, Sun B, Xu L, Liu W. Chen L, et al. Plant Signal Behav. 2016 Oct 2;11(10):e1238548. doi: 10.1080/15592324.2016.1238548. Plant Signal Behav. 2016. PMID: 27662421 Free PMC article. - Study of the grafting compatibility of the apple rootstock 12-2, resistant to apple replant diseases (ARD).
Mao Y, Cui X, Wang H, Qin X, Liu Y, Hu Y, Chen X, Mao Z, Shen X. Mao Y, et al. BMC Plant Biol. 2022 Sep 30;22(1):468. doi: 10.1186/s12870-022-03847-8. BMC Plant Biol. 2022. PMID: 36180863 Free PMC article. - High temperature perception in leaves promotes vascular regeneration and graft formation in distant tissues.
Serivichyaswat PT, Bartusch K, Leso M, Musseau C, Iwase A, Chen Y, Sugimoto K, Quint M, Melnyk CW. Serivichyaswat PT, et al. Development. 2022 Mar 1;149(5):dev200079. doi: 10.1242/dev.200079. Epub 2022 Feb 28. Development. 2022. PMID: 35217857 Free PMC article. - Transcriptome Profiling to Dissect the Role of Genome Duplication on Graft Compatibility Mechanisms in Watermelon.
Kaseb MO, Umer MJ, Anees M, Zhu H, Zhao S, Lu X, He N, El-Remaly E, El-Eslamboly A, Yousef AF, Salama EAA, Alrefaei AF, Kalaji HM, Liu W. Kaseb MO, et al. Biology (Basel). 2022 Apr 11;11(4):575. doi: 10.3390/biology11040575. Biology (Basel). 2022. PMID: 35453774 Free PMC article.
References
- Lough TJ, Lucas WJ. Integrative plant biology: Role of phloem long-distance macromolecular trafficking. Annu Rev Plant Biol. 2006;57:203–232. - PubMed
- Garner RJ, Bradley S. The grafter’s handbook. London: Mitchell Beazley; 2013. Revised & updated edition.
- Musselman LJ. The Biology of Striga, Orobanche, and other Root-Parasitic Weeds. Annual Review of Phytopathology. 1980;18:463–489.
- Turnbull CG, Booker JP, Leyser HM. Micrografting techniques for testing long-distance signalling in Arabidopsis. The Plant journal. 2002;32:255–262. - PubMed
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases